Abstract
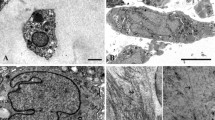
Evidence has accumulated in recent years that programmed cell death (PCD) is not necessarily synonymous with the classical apoptosis, as defined by Kerr and Wyllie, but that cells use a variety of pathways to undergo cell death, which are reflected by different morphologies. Although chondrocytes with the hallmark features of classical apoptosis have been demonstrated in culture, such cells are extremely rare in vivo. The present review focuses on the morphological differences between dying chondrocytes and classical apoptotic cells. We propose the term ‘chondroptosis’ to reflect the fact that such cells are undergoing apoptosis in a non-classical manner that appears to be typical of programmed chondrocyte death in vivo. Unlike classical apoptosis, chondroptosis involves an initial increase in the endoplasmic reticulum and Golgi apparatus, reflecting an increase in protein synthesis. The increased ER membranes also segment the cytoplasm and provide compartments within which cytoplasm and organelles are digested. In addition, destruction occurs within autophagic vacuoles and cell remnants are blebbed into the lacunae. Together these processes lead to complete self-destruction of the chondrocyte as evidenced by the presence of empty lacunae. It is speculated that the endoplasmic reticulum pathway of apoptosis plays a greater role in chondroptosis than receptor-mediated or mitochondrial pathways and that lysosomal proteases are at least as important as caspases. Because chondroptosis does not depend on phagocytosis, it may be more advantageous in vivo, where chondrocytes are isolated within their lacunae. At present the initiation factors or the molecular pathways involved in chondroptosis remain unclear.
Similar content being viewed by others
References
Kerr JF. Shrinkage necrosis: A distinct mode of cellular death. J. Patol 1971; 105: 13–20.
Wyllie AH, Kerr JFR, Currie AR. Cell death in the normal neonatal rat adrenal cortex. J Path 1973; 111: 255–261.
Kerr J.F. History of the events leading to the formulation of the apoptosis concept. Toxicology 2002; 181/182: 471–474.
Clarke PGH. Developmental cell death: Morphological diversity and multiple mechanisms. Anat Embryol 1990; 181: 195–213.
Bowen ID, Apoptosis or programmed cell death? Cell Biol Int 1993; 17: 365–380.
Schwartz LM, Smith SW, Jone MW, Osborne BA. Do all programmed cell deaths occur via apoptosis? Proc Natl Acad Sci USA 1993; 90: 980–984.
Lockshin RA, Zakeri Z. Programmed cell death: Early changes in metamorphosing cells. Biochem Cell Biol 1994; 72: 589–596.
Zakeri ZF, Bursch W, Tenniswood M, Lockshin RA. Cell death: Programmed, apoptosis, necrosis, or other? Cell Death Diff 1995; 2: 87–96.
Fukuda T, Wang H, Nakanishi H, Yamamoto K, Kosaka T. Novel non-apoptotic morphological changes in neurons of the mouse hippocampus following transient hypoxic-ischemia. Neurosci Res 1993; 33: 49–55.
Sperandio S, de B, I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci USA 2000; 97: 14376–14381.
Roach HI, Clarke, NMP. “Cell paralysis” as an intermediate stage in the programmed cell death of epiphyseal chondrocytes. J. Bone Miner Res 1999; 14: 1367–1378.
Roach HI, Clarke NMP. Physiological cell death of chondrocytes in vivo is not confined to apoptosis: New observations on the mammalian growth plate. J Bone Joint Surg (Br) 2000; 82–B; 601–613.
Leist M, Jaattela M. Four deaths and a funeral: From caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2001; 2: 589–598.
Bursch W, Ellinger A, Gerner C, Frohwein U, Schulte-Hermann R. Programmed cell death (PCD). Apoptosis, autophagic PCD, or others? Ann NY Acad Sci 2000; 926: 1–12.
Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ 2001; 8: 569–581.
Wyllie AH, Kerr JFR, Currie AR. Cell death: The significance of apoptosis. Int Rev Cytol 1980; 68: 251–303.
Majno G, Joris I. Apoptosis, onsosis, and necrosis. Am J Pathol 1995: 146: 3–15.
Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell 1997; 88: 347–354.
Horton We, Feng L, Adams C. Chondrocyte apoptosis in development, aging and disease. Matrix Biology 1998; 17: 107–115.
Peter ME, Heufelder AE, Hengartner MO. Advances in apoptosis research. Proc Natl Acad Sci USA 1997; 94: 12736–12737.
Aigner T, Kim HA. Apoptosis and cellular vitality: Issues in osteoarthritic cartilage degeneration. Arthritis Rheum 2002; 46: 1986–1996.
Van Cruchten S, Van Den BW. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol 2002; 31: 214–223.
Van Cruchten S, Van Den BW. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol 2002; 31: 214–223.
Nagata S, Nagase H, Kawane K, Mukae N, Fukuyama H. Degradation of chromosomal DNA during apoptosis. Cell Death Differ 2003; 10: 108–116.
Krieser RJ, White K. Engulfment mechanism of apoptotic cells. Curr Opin Cell Biol 2002; 14: 734–738.
Fadk VA, Chimini G. The phagocytosis of apoptotic cells. Semin Immunol 2001; 13: 365–372.
Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature 2000; 407: 784–788.
Hatori M, Klatte KJ, Teixeira CC, Shapiro IM. End labeling studies of fragmented DNA in the avian growth plate: Evidence of apoptosis in terminally differentiated chondrocytes. J Bone Joint Surg 1995; 10: 1960–1968.
Zenmyo M, Komiya S, Kawabata R, Sasaguri Y, Inoue A, Morimatsu M. Morphological and biochemical evidence for apoptosis in the terminal hypertrophic chondrocytes of the growth plate. J Path 1996; 180: 430–433.
Kouri JB, Rosales-Encina JL, Chaudhuri PP, Luna J, Mena P. Apoptosis in human osteoarthritic cartilage: A microscopy report. Med Sci Res 1997; 25: 245–248.
Aizawa T, Kokubun S, Tanaka Y. Apoptosis and proliferation of growth plate chondrocytes in rabbits. J Bone Joint Surg (Br) 1997; 79–B: 483–486.
Adams CS, Horton WE. Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec 1998; 240: 418–425.
Blanco FJ, Guitaian R, Vazquez-Martul E, DeToro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum 1998; 41: 284–289.
Fujita I, Hirata S, Ishikawa H, Mizuno K, Itoh H. Apoptosis of hypertrophic chondrocytes in rat cartilaginous growth plate. Journal of Orthopaedic Science 1997; 2: 328–333.
Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum 1998; 41: 1632–1638.
Heraud F, Heraud A, Harmand MF. Apoptosis in normal and osteoarthritic human articulate cartilage [In Process Citation]. Ann Rheum Dis 2002; 59: 959–965.
Aigner T, Hemmel M, Neureiter D, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: A study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum 2001; 44: 1304–1312.
Kim HA, Lee YJ, Seong SC, Choe KW, Song YW. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol 2000; 27: 455–462.
Wilsman NJ, Farnum CE, Hilley HD. Ultrastructural evidence of a functional heterogeneity among physeal chondrocytes in growing swine. Am J Vet Res 1981; 42: 1547–1553.
Farnum CE, Wilsman NJ. Condensation of hypertrophic chondrocytes at the chondro-osseous junction of growth plate cartilage in Yacata swine: Relationship to long bone growth. Am J Anat 1989; 186: 346–358.
Erenpreisa J, Roach HI. Aberrant death in dark chondrocytes of the avian growth plate. Cell Death Diff 1998; 5: 60–66.
Kouri-Flores JB, Abbud-Lozoya KA, Roja-Morales L. Kinetics of the ultrastructural changes in apoptotic chondrocytes from an osteoarthrosis rate model: A window of comparison to the cellular mechanism of apoptosis in human chondrocytes. Ultrastruct Pathol 2002; 26: 33–40.
Lozoya KA, Flores JB. A novel rat osteoarthrosis model to assess apoptosis and matrix degradation. Pathol Res Pract 2000; 196: 729–745.
Kouri JB, Rojas L, Perez E, Abbud-Lozoya KA. Modifications of Golgi complex in chondrocytes from osteoarthrotic (OA) rat cartilage. J Histochem Cytochem 2002; 50: 1333–1340.
Reggiori F, Klionsky DJ. Autophagy in the eukaryotic cell. Eukaryot Cell 2002; 1: 11–21.
Larsen KE, Sulzer D. Autophagy in neurons: A review. Histol Histopathol 2002; 17: 897–908.
Ogier-Denis E, Codogno P. Autophagy: A barrier or an adaptive response to cancer. Biochim Biophys Acta 2003; 1603: 113–128.
Bowen ID, Mullarkey K, Morgan SM. Programmed cell death during metamorphosis in the blow-fly Calliphora vomitoria. Microsc Res Techn 1996; 34: 202–217.
D’Herde K, De Prest B, Roels F. Subtypes of active cell death in the granulosa of ovarian atretic follicles in the quail. Reprod Nutr Dev 1996; 36: 175–189.
Uchiyama Y. Autophagic cell death and its execution by lysosomal cathepsins. Arch Histol Cytol 2001; 64: 233–246.
Lockshin RA, Zakeri Z. Caspase-independent cell deaths. Curr Opin Cell Biol 2002; 14: 727–733.
Matsuo M, Nishida K, Yoshida A, Murakami T, Inoue H. Expression of caspase-3 and-9 relevant to cartilage destruction and chondrocyte apoptosis in human osteoarthritic cartilage. Acta Med Okayama 2001; 55: 333–340.
Hashimoto S, Ochs RL, Rose F, et al. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc Natl Acad Sci USA 1998; 95: 3094–3099.
Kouri JB, Aguilera JM, Reyes J, Lozoya KA, Gonzalez S. Apoptotic chondrocytes from osteoarthrotic human articular cartilage and abnormal calcification of subchondral bone. J Rheumatol 2000; 27: 1005–1019.
Kirsch T, Wang W, Pfander D. Functional differences between growth plate apoptotic bodies and matrix vesicles. J Bone Miner Res 2003; 18: 1872–1881.
Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H, MT1–MMP-dependent, apoptotic remodeling of unmineralized cartilage: A critical process in skeletal growth. J Cell Biol 2003; 163: 661–671.
Nakagawa T, Zhu H, Morishima N, Li E, Zu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 2000; 403: 98–103.
Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 2002; 277: 34287–34294.
Stanger BZ. Looking beneath the surface: The cell death pathway of FAS/APO-1 (CD95). Mol Med 1996: 2: 7–20.
Pinkoski MJ, Green DR. Fas ligand, death gene. Cell Death Differ 1999; 6: 1174–1181.
Kroemer G. The mitochondrion as an integratior/coordinator of cell death pathways. Cell Death Diff 1998; 5: 547.
Hashimoto S, Setareh M, Ochs RL, Lotz M. The Fas/Fas ligand expression and induction of apoptosis in chondrocytes. Arthritis Rheum 1997; 40: 1749–1755.
Aizawa T, Kon T, Einhorn TA, Gerstenfeld LC. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J Orthop Res 2001; 19: 785–796.
Pettersen I, Figenschau Y, Olsen E, Bakkelund W, Smedsrod B, Sveinbjornsson B. Tumor necrosis factor-related apoptosis-inducing ligand induces apoptosis in human articular chondrocytes in vitro. Biochem Biophys Res Commun 2002; 296: 671–676.
Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol 1995: 146: 75–85.
Greisberg J, Bliss M, Terek R. The prevalence of nitric oxide in apoptotic chondrocytes of osteoarthritis. Osteoarthritis Cartilage 2002; 10: 207–211.
Kuhn K, Lotz M. Mechanisms of sodium nitroprusside-induce death in human chondrocytes. Rheumatol Int 2003; 23: 241–247.
Vu TH, Shipley JM, Bergers G, et al. MMP-9/Gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998; 93: 411–422.
Gibson G, Lin DL, Roque M. Apoptosis of terminally differentiated chondrocytes in culture. Exp Cell Res 1997; 233: 372–382.
Teixeira CC, Mansfield K. Hertkorn C, Ischiropoulos H, Shapiro IM. Phosphate-induced chondrocyte apoptosis is linked to nitric oxide generation. Am J Physiol Cell Physiol 2001; 281: C833–C839.
Mansfield K, Rajpurohit R, Shapiro IM. Extracellular phosphate ions cause apoptosis of terminally differentiated epiphyseal chondrocytes. J Cell Physiol 1999; 179: 276–286.
Gibson G, Lin DL, Wang X, Zhang I. The release and activation of transforming growth factor beta2 associated with apoptosis of chick hypertrophic chondrocytes. J Bone Miner Res 2001; 16: 2330–2338.
Dessau W, Sasse J, Timpl R, Jilek F, Von der MK. Synthesis and extracellular deposition of fibronectin in chondrocyte cultures. Response to the removal of extracellular cartilage matrix. J Cell Biol 1978; 79: 342–355.
Stupack DG, Cheresh DA. Get a ligand, get a life: Integrins, signaling and cell survival. J Cell Sci 2002; 115: 3729–3738.
Hirsch MS, Lunsford LE, Trinkhaus-Randall V, Svoboda KKH. Chondrocyte survival and differentiation in situ are integrin mediated. Dev Dynam 1997; 210: 249–263.
Cao L, Lee V, Adams ME, Kiani C, Zhang Y, Hu W, Yang BB. beta-Integrin-collagen interaction reduces chondrocyte apoptosis. Matrix Biol 1999; 18: 343–355.
Yang C, Li SW, Helminen HJ, Khillan JS, Bao Y, Prockop DJ. Apoptosis of chondrocytes in transgenic mice lacking collagen II. Exp Cell Res 1997; 235: 370–373.
Gibson G. Active rol of chondrocyte apoptosis in endochondral ossification. Micros Res Tech 1998; 43: 191–204.
Gibson GJ, Kohler W, Schaffler MB. Chondrocyte apoptosis in endochondral ossification of chick sterna. Dev Dynam 1995; 203: 468–476.
Hwang WS. Ultrastructure of human foetal and neonatal hyaline cartilage. J Path 1978; 126: 209–214.
Hwang WS, Tock EPC, Tan KL, Tan LKA. The pathology of cartilage in chondrodysplasias. J Path 1979: 127: 11–18.
Unger RH, Orci L. Lipoapoptosis: Its mechanism and its diseases. Biochim Biophys Acta 2002; 1585: 202–212.
Erenpreisa J, Roach HI. Epigenetic selection as a possible component of transdifferentiation. Further study of the commitment of hypertrophic chondrocytes to become osteocytes. Mech Age Dev 1996; 87: 165–182.
Roach HI, Clarke NMP. Programmed cell death of chondrocytes in vivo: Apoptosis dark chondrocytes, and cell paralysis. 2000, 325–330.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roach, H.I., Aigner, T. & Kouri, J.B. Chondroptosis: A variant of apoptotic cell death in chondrocytes?. Apoptosis 9, 265–277 (2004). https://doi.org/10.1023/B:APPT.0000025803.17498.26
Issue Date:
DOI: https://doi.org/10.1023/B:APPT.0000025803.17498.26