Abstract
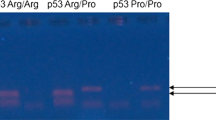
Infection of high risk human papillomaviruses (HPVs) specifically the types 16 and 18 has been strongly implicated in the development of cervical cancer. The E6 oncoproteins of these high risk HPVs are known to bind and induce degradation of p53 tumour suppressor protein through the ubiquitin pathways. This degradation is controlled by a common polymorphism of the p53 gene encoding either a proline or an arginine at its codon 72 in exon 4. Recently, it has been demonstrated that the presence of homozygous arginine at codon 72 renders p53 about seven times more susceptible to E6-mediated proteolytic degradation as well as to cervical cancer than those with proline homozygotes or proline/arginine heterozygotes. In India, prevalence of HPV as well as cancers of the uterine cervix and the oral cavity are highest in the world. We have examined this allele-specific predisposition in cervical and oral cancer which is associated with HPV as well as in a non-HPV-linked cancer of the breast. We have carried out investigation in women comprising whole spectrum of cervical lesions with 128 HPV 16/18 positive and 35 HPV negative invasive cervical carcinomas and 34 cases of HPV (16/18) positive and 16 HPV negative cervical dysplasias (mild, moderate and severe) and 104 age-group-matched healthy women as controls. Additionally, we have analysed p53Arg-Pro polymorphism in 13 high risk HPV positive and 31 HPV negative oral cancers along with 20 normal controls and 77 breast cancers with 41 age-matched healthy controls.
We observed more than 2 fold higher risk for homozygous arginine (χ2 = 6.3, df = 2, p = 0.04; OR = 2.3; 95% CI: 1.08–5.16) for HPV 16/18-positive cervical carcinomas when comparison was made only between HPV positive cervical cancers and normal controls but most interestingly, no significant association either in the frequency of homozygous arginine or proline alleles or their heterozygotes could be observed when all the three groups i.e. HPV-positive, HPV-negative cervical cancers and controls were considered simultaneously. No difference was also observed for either arginine or proline polymorphism between women with precancerous lesions of the uterine cervix carrying HPV 16/18 infection and controls. Similarly, increased risk of oral or breast cancer could not be correlated with the polymorphism of arginine/proline allele.
Thus the interaction between HPV oncoproteins and the p53 gene polymorphism specifically, homozygous arginine at codon 72 appears to play no role in the development of either cervical or oral cancer and also it can not serve as a biomarker for early identification of cervical, oral or breast cancer.
Similar content being viewed by others
References
Luthra UK, Prabhakar AK, Seth P, Agarwal SS, Murthy NS, Bhatnagar P, Das DK, Sharma BK: Natural history of precancerous and early cancerous lesions of uterine cervix. Acta Cytol 31: 226-234, 1987
Murthy NS, Juneja A, Sehgal A, Prabhakar AK, Luthra UK: Cancer projection by the turn of century-Indian science. Ind J Cancer 27: 74-82, 1990
WHO (World Health Organization). Control of cancer of the cervix uteri. A WHO meeting. Bull WHO 64: 607-618, 1986
zur Hausen H: Papillomavirus infections — a major cause of human cancers. Biochim Biophys Acta 1288: 55-78, 1996
IARC: The evaluation of carcinogenic risks in humans. Human papillomaviruses. IARC, Lyon 64, 1995
Vousden KH: Human papillomaviruses and cervical carcinoma: Cancer Cells 1: 43-50, 1989
Barbosa MS, Schlegel R: The E6 and E7 genes of HPV-18 are sufficient for inducing two-stage in vitro transformation of human keratinocytes. Oncogene 4: 1529-1532, 1989
Hawley NP, Vousden KH, Hubbert NL, Lowy DR, Schiller JT: HPV 16 E6 and E7 proteins cooperate to immortalize of human foreskin keratinocytes. EMBO J 8: 3905-3910, 1989
Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R: The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol 63: 4417-4421, 1989
Tanaka A, Noda T, Yajima H, Hatanaka M, Ito Y: Identification of a transforming gene of human papillomavirus type 16. J Virol 63: 1465-1469, 1989
Halbert CL, Demers GW, Galloway DA: The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol 65: 473-478, 1991
Crook T, Morgenstern JP, Crawford L, Banks L: Continued expression of HPV-16 E7 protein is required for maintenance of the transformed phenotype of cells co-transformed by HPV 16 plus EJ-ras. EMBO J 8: 513-519, 1989
Werness BA, Levine AJ, Howley PM: Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248: 76-79, 1990
Dyson N, Howley PM, Munger K, Harlow E: The human papilloma virus-16 E7 oncoprotein is able to bind to the retrinoblastoma gene product. Science 243: 934-937, 1989
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM: The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63: 1129-1136, 1990
Crook T, Wrede D, Vousden KH: p53 point mutation in HPV negative human cervical carcinoma cell lines. Oncogene 6: 873-875, 1991
Scheffner M, Munger K, Byrne JC, Howley PM: The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA 88: 5523-5527, 1991
Vogelstein B, Kinzler KW: p53 Function and dysfunction. Cell 70: 523-526, 1992
Matlashewski GJ, Tuck S, Pim D, Lamb P, Schneider J, Crawford LV: Primary structure polymorphism at amino acid residue 72 of human p53. Mol Cell Biol 7: 961-963, 1987
Beckman G, Birgander R, Sjalander A, Saha N, Holmberg PA, Kivela A Beckman L: Is p53 polymorphism maintained by natural selection? Hum Hered 44: 266-270, 1994
Storey A, Thomas M, Kalita A, Harwood C, Gardiol D, Mantovani F, Breuer J, Leigh IM, Matlashewski G, Banks L: Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature 393: 229-234, 1998
Rosenthal AN, Ryan A, Al-Jehani RM, Storey A, Harwood CA, Jacobs IJ: p53 codon 72 polymorphism and risk of cervical cancer in UK. Lancet 352: 871-872, 1998
Helland A, Langerod A, Johnsen H, Olsen AO, Skovlund E, Borresen-Dale AL: p53 Polymorphism and risk of cervical cancer. Nature 396: 530-531, 1998
Josefsson AM, Magnusson PK, Ylitalo N, Quarforth-Tubbin P, Ponten J, Adami HO, Gyllensten UB: p53 Polymorphism and risk of cervical cancer. Nature 396: 531, 1998
Minaguchi T, Kanamori Y, Matsushima M, Yoshikawa H, Taketani Y, Nakamura Y: No evidence of correlation between polymorphism at codon 72 of p53 and risk of cervical cancer in Japanese patients with human papillomavirus 16/18 infection. Cancer Res 58: 4585-4586, 1998
Hildesheim A, Schiffman M, Brinton LA, Fraumeni JF Jr, Herrero R, Bratti MC, Schwartz P, Mortel R, Barnes W, Greenberg M, McGowan L, Scott DR, Martin M, Herrera JE, Carrington M: p53 polymorphism and risk of cervical cancer. Nature 396: 531-532, 1998
Lanham S, Campbell I, Watt P, Gornall R: p53 Polymorphism and risk of cervical cancer. Lancet 352: 1631, 1998
Giannoudis A, Graham DA, Southern SA, Herrington CS: p53 codon 72 ARG/PRO polymorphism is not related to HPV type or lesion grade in low-and high-grade squamous intra-epithelial lesions and invasive squamous carcinoma of the cervix. Int J Cancer 83: 66-69, 1999
Klaes R, Ridder R, Schaefer U, Benner A, von Knebel Doeberitz M: No evidence of p53 allele-specific predisposition in human papillomavirus-associated cervical cancer. J Mol Med 77: 299-302, 1999
Hayes VM, Hofstra RM, Buys CH, Hollema H, van der Zee AG: Homozygous arginine-72 in wild type p53 and risk of cervical cancer. Lancet 352: 1756, 1998
Yamashita T, Yaginuma Y, Saitoh Y, Kawai K, Kurakane T, Hayashi H, Ishikawa M: Codon 72 polymorphism of p53 as a risk factor for patients with human papillomavirus-associated squamous intraepithelial lesions and invasive cancer of the uterine cervix. Carcinogenesis 20: 1733-1736, 1999
Wang YC, Lee HS, Chen SK, Chang YY, Chen CY: Prognostic significance of p53 codon 72 polymorphism in lung carcinomas. Eur J Cancer 35: 226-230, 1999
Zehbe I, Voglino G, Wilander E, Genta F, Tommasino M: Codon 72 polymorphism of p53 and its association with cervical cancer. Lancet 354: 218-219, 1999
Peller S, Halperin R, Schneider D, Kopilova Y, Rotter V: Polymorphisms of the p53 gene in women with ovarian or endometrial carcinoma. Oncol Rep 6: 193-197, 1999
Murata M, Tagawa M, Kimura H, Kakisawa K, Shirasawa H, Fujisawa T: Correlation of the mutation of p53 gene and the polymorphism at codon 72 in smoking-related non-small cell lung cancer patients. Int J Oncol 12: 577-581, 1998
Cuzick J, Terry G, Ho L, Hollingworth T, Anderson M: Human papillomavirus type 16 in cervical smears as predictor of high-grade cervical intraepithelial neoplasia. Lancet 339: 959-960, 1992
Das BC, Sharma JK, Gopalkrishna V, Das DK, Singh V, Gissmann L, zur Hausen H, Luthra, UK: A high frequency of human papillomavirus DNA sequences in cervical carcinomas of Indian women as revealed by Southern blot hybridization and polymerase chain reaction. J Med Virol 36: 239-245, 1992a
Balaram P, Nalinakumari KR, Abraham E, Balan A, Hareendran NK, Bernard HU, Chan SY: Human papillomaviruses in 91 oral cancers from Indian betel quid chewers-high prevalence and multiplicity of infections. Int J Cancer 61: 450-454, 1995
Wrede D, Luqmani YA, Coombes RC, Vousden KH: Absence of HPV 16 and 18 DNA in breast cancer. Br J Cancer 65: 891-894, 1992
Gopalkrishna V, Singh UR, Sodhani P, Sharma JK, Hedau ST, Mandal AK, Das BC: Absence of human papillomavirus DNA in breast cancer as revealed by polymerase chain reaction. Breast Cancer Res Treat 39: 197-202, 1996
Das BC, Sharma JK, Gopalkrishna V, Luthra UK: Analysis by polymerase chain reaction of the physical state of human papillomavirus type 16 DNA in cervical preneoplastic and neoplastic lesions J Gen Virol 73: 2327-2336, 1992b
Maniatis T, Fritsch EF, Sambrook J: In: Molecular Cloning — A laboratory Manual. Cold Spring Harbor Laboratory, 1989
van Duin M, Snijders PJ, Vossen MT, Klaassen E, Voorhorst F, Verheijen RH, Helmerhorst TJ, Meijer CJ, Walboomers JM: Analysis of human papillomavirus type 16 E6 variants in relation to P53 codon 72 polymorphism genotypes in cervical carcinogenesis. J Gen Virol 81: 317-325, 2000
Nagpal JK, Patnaik S, Das BR: Prevalence of high-risk human papilloma virus types and its association with p53 codon 72 polymorphism in tobacco addicted oral squamous cell carcinoma (OSCC) patients of Eastern India. Int J Cancer 97: 649-653, 2002
Tandle AT, Sanghvi V, Saranath D: Determination of p53 genotypes in oral cancer patients from India. Br J Cancer 84: 739-742, 2001
Kuo MY, Huang JS, Hsu HC, Chiang CP, Kok SH, Kuo YS, Hong CY: Infrequent p53 mutations in patients with areca quid chewing-associated oral squamous cell carcinomas in Taiwan. J Oral Pathol Med 28: 221-225, 1999
Weston A, Godbold JH: Polymorphisms of H-ras-1 and p53 in breast cancer and lung cancer: A meta-analysis. Environ Health Perspect 105(suppl 4): 919-926, 1997
Weston A, Caporaso NE, Perrin LS, Sugimura H, Tamai S, Krontiris TG, Trump BF, Hoover RN, Harris CC: Relationship of H-ras-1, L-myc, and p53 polymorphisms with lung cancer risk and prognosis. Environ Health Perspect 98: 61-67, 1992
Jin X, Wu X, Roth JA, Amos CI, King TM, Branch C, Honn SE, Spitz MR: Higher lung cancer risk for younger African-Americans with the Pro/Pro p53 genotype carcinogenesis 16: 2205-2208, 1995
Tagawa M, Murata M, Kimura H: Prognostic value of mutations and a germ line polymorphism of the p53 gene in non-small cell lung carcinoma: Association with clinicopathological features. Cancer Lett 128: 93-99, 1998
Mittra I, Badwe RA, Desai PB, Yeole BB, Jussawala DJ: Early detection of breast cancer in developing countries. Lancet 1: 710-720, 1989
NCRP (National Cancer Registry Programme): Indian Council of Medical Research, 2001
Buller RE, Sood A, Fullenkamp C, Sorosky J, Powills K, Anderson B: The influence of the p53 codon 72 polymorphism on ovarian carcinogenesis and prognosis. Cancer Gene Ther 4: 239-245, 1997
de la Calle-Martin O, Fabregat V, Romero M, Soler J, Vives J, Yague J: AccII polymorphism of the p53 gene. Nucleic Acids Res 18: 4963, 1990
Kaelbling M, Burk RD, Atkin NB, Johnson AB, Klinger HP: Loss of heterozygosity on chromosome 17p and mutant p53 in HPV-negative cervical carcinomas. Lancet 340: 140-142, 1992
Mullokandov MR, Kholodilov NG, Atkin NB, Burk RD, Johnson AB, Klinger HP: Genomic alterations in cervical carcinoma: Losses of chromosome heterozygosity and human papilloma virus tumor status. Cancer Res 56: 197-205, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Katiyar, S., Thelma, B., Murthy, N. et al. Polymorphism of the p53 codon 72 arg/pro and the risk of HPV type 16/18-associated cervical and oral cancer in India. Mol Cell Biochem 252, 117–124 (2003). https://doi.org/10.1023/A:1025546610920
Issue Date:
DOI: https://doi.org/10.1023/A:1025546610920