Abstract
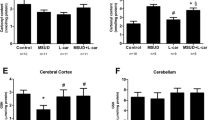
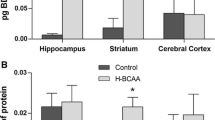
Maple syrup urine disease (MSUD) is an inherited metabolic disorder biochemically characterized by the accumulation of branched-chain amino acids (BCAAs) and their branched-chain keto acids (BCKAs) in blood and other tissues. Neurological dysfunction is usually present in the affected patients, but the mechanisms of brain damage in this disease are not fully understood. Considering that brain energy metabolism seems to be altered in MSUD, the main objective of this study was to investigate the in vitro effect of BCAAs and BCKAs on creatine kinase activity, a key enzyme of energy homeostasis, in brain cortex of young rats. BCAAs, but not their BCKAs, significantly inhibited creatine kinase activity at concentrations similar to those found in the plasma of MSUD patients (0.5–5 mM). Considering the crucial role creatine kinase plays in energy homeostasis in brain, if this effect also occurs in the brain of MSUD patients, it is possible that inhibition of this enzyme activity may contribute to the brain damage found in this disease.
Similar content being viewed by others
REFERENCES
Menkes J. H. 1959. Maple syrup urine disease: Isolation and identification of organic acids in the urine. Pediatrics 23:348–353.
Dancis, J., Hutzler, J., and Levitz, M. 1960. Metabolism of the white blood cells in maple syrup urine disease. Biochim. Biophys. Acta 43:342–347.
Chuang D. T. and Shih, V. E. 2001. Maple syrup urine disease (branched-chain ketoaciduria). Pages 1667-1724, in Scriver, C. R., Beaudet, A. L., Sly, W. S., and Valle, D. (eds), The Metabolic & Molecular Bases of Inherited Diseases, 8th ed., McGraw-Hill, New York.
Kill, R. and Rokkones, T. 1964. Late manifesting variant of branched-chain ketoaciduria (maple syrup urine disease). Acta Paediatr. 53:356–361.
Riviello, J. J., Jr., Rezvani, I., Digeorge, A. M., and Foley, C. M. 1991. Cerebral edema causing death in children with maple syrup urine disease. J. Pediatr. 119:42––45.
Kamei, A., Takashima, S., Chan, F., Becker, L. E. 1992. Abnormal dendritic development in maple syrup urine disease. Pediatr. Neurol. 8:145–147.
Wallimann, T., Wyss, M., Brdiczka, D., and Nicolay, K. 1992. Intracellular compartmentation, structure and function of creatine kinase in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 281:21–40.
Wyss, M., Smeitink, J., Wevers, R. A., and Wallimann, T. 1992. Mitochondrial creatine kinase: A key enzyme of aerobic energy metabolism. Biochim. Biophys. Acta 1102:119–166.
Tomimoto, H., Yamamoto, K., Homburger H. A., and Yanagihara, T. 1993. Immunoelectron microscopic investigation of creatine kinase BB-isoenzyme after cerebral ischemia in gerbils. Acta Neuropathol. 86:447–455.
David, S. S., Shoemaker, M., and Haley, B. E. 1998. Abnormal properties of creatine kinase in Alzheimer's disease brain: Correlation of reduced enzyme activity and active site photo-labelling with aberrant cytosol-membrane parttitioning. Mol. Brain Res. 54:276–287.
Aksenov, M., Aksenova, M., Butterfield, A. D., and Markesbery, W. R. 2000. Oxidative modification of creatine kinase BB in Alzheimer's disease brain. J. Neurochem. 74:2520-2527.
Aksenov, M., Aksenova, M., Payne, R. M., Trojanovski, J. Q., Schmidt, K. L., Carney, J. M., Butterfield, D. A., and Markesbery, W. R. 1999. Oxidation of cytosolic proteins and expression of creatine kinase BB in frontal lobes of neurodegenerative disorders. Dement. Geriatr. Cogn. Disord. 10:158–165.
Hughes, B. P. 1962. A method for estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathological sera. Cin. Chim. Acta 7:597–603.
Lowry O. H., Rosebrough N. J., Farr A. L., and Randal R. J. 1951. Protein measurement with a Folin phenol reagent. J. Biol. Chem. 193:265–275.
Zielke, H. R., Zielke, C. L., Baab, P. J., and Collins, R. M. 2002. Large neutral amino acids auto exchange when infused by microdialysis into the rat brain: Implication for maple syrup urine disease and phenylketonuria. Neurochem. Int. 40:347–354.
Araujo, P., Wasserman, G. F., Tallini, K., Furlanetto, V., Vargas, C. R., Wannmacher, C. M., Dutra-Filho, C. S., Wyse, A. T. S., and Wajner, M. 2001. Reduction of large neutral amino acids levels in plasma and brain of hyperleucinemic rats. Neurochem. Int. 38:529–537.
Fontella, F. U., Gassen, E., Pulrolnik, V., Wannmacher, C. M. D., Klein, A. B., Wajner, M., and Dutra-Filho, C. S. 2002. Stimulation of lipid peroxidation in vitro in rat brain by the metabolites accumulating in maple syrup urine disease. Metab. Brain Dis. 17:47–54.
Zielke, H. R., Huang, Y., Baab, P. J., Collins, R. M., Jr., Zielke, C. L., and Tildon, J. T. 1997. Effecte of alpha-ketoisocaproate and leucine on the in vivo oxidation of glutamate and glutamine in the rat brain. Neurochem. Res. 22:1159-1164.
Tavares, R. G., Santos, C. E., Tasca, C. I., Wajner, M., Souza, D. O., and Dutra-Filho, C. S. 2000. Inhibition of glutamate uptake into synaptic vesicles of rat brain by the metabolites accumulating in maple syrup urine disease. J. Neurol. Sci. 181:44–49.
Reis, M., Farage, M., and Wolosker, H. 2000. Chloride-dependent inhibition of vesicular glutamate uptake by by alpha-keto acids accumulated in maple syrup urine disease. Biochim. Biophys. Acta 1475:114–118.
Jouvet, P., Rustin, P., Taylor, D. L., Pocock, J. M., Felderhoff-Mueser, U., Mazarakis, N. D., Sarraf, C., Joashi, U., Kozma, M., Greenwood, K., Edwards, A. D., and Mehemet, A. 2000. Branched chain amino acids induce apoptosis in neural cells without mitochondrial membrane depolarization or cytochrome c release: Implications for neurological impairment associated with maple syrup urine disease. Mol. Biol. Cell. 11:1919-1932.
Jouvet, P., Kozma, M., and Mehemet, H. 2000. Primary human fibroblasts from a maple syrup urine disease patient undergo apoptosis following exposure to physiological concentrations of branched chain amino acids. Ann. N. Y. Acad. Sci. 926:116–121.
Coitinho, A. S., de Mello, C. F., Lima, T. T., de Bastiani, J., Fighera, M. R., and Wajner, M. 2001. Pharmacological evidence that alpha-keto isovaleric acid induces convulsions through GABAergic and glutamatergic mechanisms in rats. Brain Res. 894:68–73.
Yudkoff, M. 1997. Brain metabolism of branched-chain amino acids. Glia 21:92–98.
Prensky, A. L. and Moser, H. W. 1966. Brain lipids, proteolipids, and free amino acids in maple syrup urine disease. J. Neurochem. 13:863–874.
Zielke, H. R., Huang, Y., and Tildon, J. T. 1996. Elevation of amino acids in the interstitial space of the rat brain following infusion of a large neutral amino acid and keto acids by microdialysis: Alpha-ketocaproate infusion. Dev. Neurosci. 18:420–425.
Saks, V. A., Ventura-Clapier, R., and Aliev, M. K. 1996. Metabolic control and metabolic capacity: Two aspects of creatine kinase functioning in the cells. Biochim. Biophys. Acta 1274:81–88.
Wallimann, T., Dolder, M., Schlattner, U., Eder, M., Hornemann, T., O'Gorman, E., Ruck, E., and Brdiczka, D. 1998. Some new aspects of creatine kinase (CK): Compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology. Biofactors 8:229–234.
Walliman, T., Dolder, M., Schlattner, U., Eder, M., Hornemann, T., Kraft, T., and Stolz, M. 1998. Creatine kinase: An enzyme with a central role in cellular energy metabolism. MAGMA 6:116–119.
Aksenov, M. Y., Aksenova, M. V., Payne, R. M., Smith, C. D., Markesbery, W. R., and Carney, J. M. 1997. The expression of creatine kinase isoenzymes in neocortex of patients with neurodegenerative disorders: Alzheimer's and Pick's disease. Experim. Neurol. 146:458–465.
Hensley, K., Hall, N., Subramaniam, R., Cole, P., Harris, M., Aksenova, M. V., Aksenov, M. Y., Gabbita, S. P., Carney, J. M., Lowell, M., Markesbery, W. R., and Butterfield, D. A. 1995. Brain regional correspondence between Alzheimer's disease histopathology biomarkers of protein oxidation. J. Neurochem. 65:2146-2156.
Brustovetsky, N., Brustovetsky, T., and Dubinsky, J. M. 2001. On the mechanisms of neuroprotection by creatine and phosphocreatine. J. Neurochem. 76:425–434.
Manos P., Bryan G. K., and Edmond J. 1991. Creatine kinase activity in postnatal rat brain development and in cultured neurons, astrocytes, and oligodendrocytes. J. Neurochem. 56:2101-2107.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pilla, C., de Oliveira Cardozo, R.F., Severo Dutra-Filho, C. et al. Creatine Kinase Activity from Rat Brain Is Inhibited by Branched-Chain Amino Acids in Vitro . Neurochem Res 28, 675–679 (2003). https://doi.org/10.1023/A:1022876130038
Issue Date:
DOI: https://doi.org/10.1023/A:1022876130038