Abstract
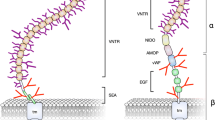
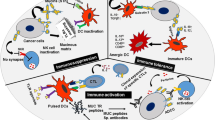
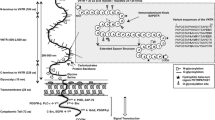
Many carcinoma-associated markers are glycoconjugates whose expression undergoes temporal or spatial regulation. Mucin-1 (MUC1), discovered through monoclonal antibody technology, is a well-documented example of such a molecule and influences numerous pathophysiological behaviors, such as the invasion and metastasis of carcinoma cells. Levels of MUC1 expression in carcinomas correlate with the clinical stage of the cancer and inversely correlate with the survival prospects of patients. The MUC1 immune response is known to provide a protective host defense mechanism against cancer. The multiple functions of MUC1 in carcinoma-host interactions are believed to be dependent on the polymorphic nature of MUC1, particularly its glycosylation status.
Similar content being viewed by others
References
Hilkens J, Kroezen V, Bonfrer JM, De Jong-Bakker M, Bruning PF, MAM-6 antigen, a new serum marker for breast cancer monitoring, Cancer Res 46, 2582–7 (1986).
Burchell J, Durbin H, Taylor-Papadimitriou J, Complexity of expression of antigenic determinants, recognized by monoclonal antibodies HMFG-1 and HMFG-2, in normal and malignant human mammary epithelial cells, J Immunol 131, 508–13 (1983).
Gendler SJ, Lancaster CA, Taylor PJ, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D, Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem 265, 15286–93 (1990).
Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA, Cloning and sequencing of a human pancreatic tumor mucin cDNA, J Biol Chem 265, 15294–9 (1990).
Ligtenberg MJ, Vos HL, Gennissen AM, Hilkens J, Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini, J Biol Chem 265, 5573–8 (1990).
Wreschner DH, Hareuveni M, Tsarfaty I, Smorodinsky N, Horev J, Zaretsky J, Kotkes P, Weiss M, Lathe R, Dion A, et al., Human epithelial tumor antigen cDNA sequences. Differential splicing may generate multiple protein forms, Eur J Biochem 189, 463–73 (1990).
Barnd DL, Lan MS, Metzgar RS, Finn OJ, Specific, major histocompatibility complex-unrestricted recognition of tumorassociated mucins by human cytotoxic T cells, Proc Natl Acad Sci USA 86, 7159–63 (1989).
Jerome KR, Barnd DL, Bendt KM, Boyer CM, Taylor-Papadimitriou J, McKenzie IF, Bast RC, Jr, Finn OJ, Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells, Cancer Res 51, 2908–16 (1991).
Jerome KR, Domenech N, Finn OJ, Tumor-specific cytotoxic T cell clones from patients with breast and pancreatic adenocarcinoma recognize EBV-immortalized B cells transfected with polymorphic epithelial mucin complementary DNA, J Immunol 151, 1654–62 (1993).
von Mensdorff-Pouilly S, Verstraeten AA, Kenemans P, Snijdewint FGM, Kok A, van Kamp GJ, Paul MA, van Diest PJ, Meijer S, Hilgers J, Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin (MUC1), J Clin Oncol 18, 574–83 (2000).
Matsushita Y, Hoff SD, Nudelman ED, Otaka M, Hakomori S, Ota DM, Cleary KR, Irimura T, Metastatic behavior and cell surface properties of HT-29 human colon carcinoma variant cells selected for their differential expression of sialyl-dimeric Le(x)-antigen, Clin Exp Metastasis 9, 283–99 (1991).
Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T, MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma, Gastroenterology 106, 353–61 (1994).
Fujita K, Denda K, Yamamoto M, Matsumoto T, Fujime M, Irimura T, Expression of MUC1 mucins inversely correlated with postsurgical survival of renal cell carcinoma patients, Brit J Cancer 80, 301–8 (1999).
Gum JR, Byrd JC, Hicks JW, Toribara NW, Lamport DT, Kim YS, Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism, J Biol Chem 264, 6480–7 (1989).
Gum JR, Hicks JW, Swallow DM, Lagace RL, Byrd JC, Lamport DT, Siddiki B, Kim YS, Molecular cloning of cDNAs derived from a novel human intestinal mucin gene, Biochem Biophys Res Commun 171, 407–15 (1990).
Porchet N, Nguyen VC, Dufosse J, Audie JP, Guyonnet-Duperat V, Gross MS, Denis C, Degand P, Bernheim A, Aubert JP, Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs, Biochem Biophys Res Commun 175, 414–22 (1991).
Meezaman D, Charles P, Daskal E, Polymeropoulos MH, Martin BM, Rose MC, Cloning and analysis of cDNA encoding a major airway glycoprotein, human tracheobronchial mucin (MUC5), J Biol Chem 269, 12932–9 (1994).
Dufosse J, Porchet N, Audie JP, Guyonnet Duperat V, Lame A, Van-Seuningen I, Marrakchi S, Degand P, Aubert JP, Degenerate 87-base-pair tandem repeats create hydrophilic/hydrophobic alternating domains in human mucin peptides mapped to 11p15, Biochem J 293, 329–37 (1993).
Toribara NW, Roberton AM, Ho SB, Kuo WL, Gum E, Hicks JW, Gum JR, Jr, Byrd JC, Siddiki B, Kim YS, Human gastric mucin. Identification of a unique species by expression cloning, J Biol Chem 268, 5879–85 (1993).
Bobek LA, Tsai H, Biesbrock AR, Levine MJ, Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7), J Biol Chem 268, 20563–9 (1993).
Shankar V, Pichan P, Eddy RL, Jr, Tonk V, Nowak N, Sait SN, Shows TB, Schultz RE, Gotway G, Elkins RC, Gilmore MS, Sachdev GP, Chromosomal localization of a human mucin gene (MUC8) and cloning of the cDNA corresponding to the carboxy terminus, Am J Respir Cell Mol Biol 16, 232–41 (1997).
Lapensee L, Paquette Y, Bleau G, Allelic polymorphism and chromosomal localization of the human oviductin gene (MUC9). Fertil Steril 68, 702–8 (1997).
Williams SJ, McGuckin MA, Gotley DC, Eyre HJ, Sutherland GR, Antalis TM, Two novel mucin genes down-regulated in colorectal cancer identified by differential display, Cancer Res 59, 4083–9 (1999).
Ligtenberg MJ, Kruijshaar L, Buijs F, van MM, Litvinov SV, Hilkens J, Cell-associated episialin is a complex containing two proteins derived from a common precursor, J Biol Chem 267, 6171–7 (1992).
Moniaux N, Nollet S, Porchet N, Degand P, Laine A, Aubert JP, Complete sequence of the human mucin MUC4: a putative cell membrane associated mucin, Biochem J 338, 325–33 (1999).
Moniaux N, Escande F, Batra SK, Porchet N, Laine A, Aubert JP, Alternative splicing generates a family of putative secreted and membrane associated MUC4 mucins, Eur J Biochem 267, 4536– 44 (2000).
Zotter S, Lossnitzer A, Hageman PC, Delemarre JF, Hilkens J, Hilgers J, Immunohistochemical localization of the epithelial marker MAM-6 in invasive malignancies and highly dysplastic adenomas of the large intestine, Lab Invest 57, 193–9 (1987).
Fontenot JD, Tjandra N, Bu D, Ho C, Montelaro RC, Finn OJ, Biophysical characterization of one-, two-, and three-tandem repeats of human mucin (muc-1) protein core, Cancer Res 53, 5386–94 (1993).
Jentoft N, Why are proteins O-glycosylated? Trends Biochem Sci 15, 291–4 (1990).
Swallow DM, Gendler S, Griffiths B, Corney G, Taylor-Papadimitriou J, Bramwell ME, The human tumour-associated epithelial mucins are coded by an expressed hypervariable gene locus PUM, Nature 328, 82–4 (1987).
Muller S, Alving K, Peter-Katalinic J, Zachara N, Gooley AA, Hanisch FG, High density O-glycosylation on tandem repeat peptide from secretory MUC1 of T47D breast cancer cells, J Biol Chem 274, 18165–72 (1999).
Taylor-Papadimitriou J, Finn OJ, Biology, biochemistry and immunology of carcinoma-associated mucins, Immunol Today 18, 105–7 (1997).
Burchell J, Gendler S, Taylor-Papadimitriou J, Girling A, Lewis A, Millis R, Lamport D, Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin, Cancer Res 47, 5476–82 (1987).
Lloyd KO, Burchell J, Kudryashov V, Yin B, Taylor PJ, Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells, J Biol Chem 271, 33325–34 (1996).
Karsten U, Diotel C, Klich G, Paulsen H, Goletz S, Muller S, Hanisch FG, Enhanced binding of antibodies to the DTR motif of MUC1 tandem repeat peptide is mediated by site-specific glycosylation, Cancer Res 58, 2541–9 (1998).
Dilulio NA, Bhavanandan VP, The saccharides of the MUC1 mucin-type glycoprotein, epitectin, produced by H.Ep.2 cells in the presence of aryl-N-acetyl-alpha-galactosaminides, Glycobiology 5, 195–9 (1995).
Bhavanandan VP, Zhu Q, Yamakami K, Dilulio NA, Nair S, Capon C, Lemoine J, Fournet B, Purification and characterization of the MUC1 mucin-type glycoprotein, epitectin, from human urine: structures of the major oligosaccharide alditols, Glycoconj J 15, 37–49 (1998).
Clausen H, Bennett EP, A family of UDP-GalNAc: polypeptide N-acetylgalactosaminyl-transferases control the initiation of mucin-type O-linked glycosylation, Glycobiology 6, 635–46 (1996).
Bennett EP, Hassan H, Mandel U, Mirgorodskaya E, Roepstorff P, Burchell J, Taylor-Papadimitriou J, Hollingsworth MA, Merkx G, van Kessel AG, Eiberg H, Steffensen R, Clausen H, Cloning of a human UDP-N-acetyl-alpha-D-Galactosamine: polypeptide N-acetylgalactosaminyltransferase that complements other Gal-NAc-transferases in complete O-glycosylation of the MUC1 tandem repeat, J Biol Chem 273, 30472–81 (1998).
Wandall HH, Hassan H, Mirgorodskaya E, Kristensen AK, Roepstorff P, Bennett EP, Nielsen PA, Hollingsworth MA, Burchell J, Taylor-Papadimitriou J, Clausen H, Substrate specificities of three members of the human UDP-N-acetylalpha-D-galactosamine: Polypeptide N-acetylgalactosaminyltransferase family, GalNAc-Ti,-T2, and-T3, J Biol Chem 272, 23503–14 (1997).
Hanisch FG, Muller S, Hassan H, Clausen H, Zachara N, Gooley AA, Paulsen H, Alving K, Peter-Katalinic J, Dynamic epigenetic regulation of initial O-glycosylation by UDP-NAcetylgalactosamine: Peptide N-acetylgalactosaminyltransferases. Site-specific glycosylation of MUC1 repeat peptide influences the substrate qualities at adjacent or distant Ser/Thr positions, J Biol Chem 274, 9946–54 (1999).
Iida S, Takeuchi H, Hassan H, Clausen H, Irimura T, Incorporation of N-acetylgalactosamine into consecutive threonine residues in MUC2 tandem repeat by recombinant human N-acetyl-D-galactosamine transferase-T1, T2 and T3, FEBS Lett 449, 230–4 (1999).
Iida S, Takeuchi H, Kato K, Yamamoto K, Irimura T, Order and maximum incorporation of N-acetyl-D-galactosamine into threonine residues of MUC2 core peptide with microsome fraction of human-colon-carcinoma LS174T cells, Biochem J 347, 535–42 (2000).
Irimura T, Denda K, Iida S, Takeuchi H, Kato K, Diverse glycosylation of MUC1 and MUC2: potential significance in tumor immunity, J Biochem (Tokyo) 126, 975–85 (1999).
Hanisch FG, Muller S, MUC1: the polymorphic appearance of a human mucin. Glycobiology 10, 439–49 (2000).
Kitamura H, Yonezawa S, Tanaka S, Kim YS, Sato E, Expression of mucin carbohydrates and core proteins in carcinomas of the ampulla of Vater: their relationship to prognosis, Jpn J Cancer Res 87, 631–40 (1996).
Walsh MD, Hohn BG, Thong W, Devine PL, Gardiner RA, Samaratunga ML, McGuckin MA, Mucin expression by transitional cell carcinomas of the bladder, Br J Urol 73, 256–62 (1994).
Masaki Y, Oka M, Ogura Y, Ueno T, Nishihara K, Tangoku A, Takahashi M, Yamamoto M, Irimura T, Sialylated MUC1 mucin expression in normal pancreas, benign pancreatic lesions, and pancreatic ductal adenocarcinoma, Hepatogastroenterology 46, 2240–5 (1999).
Dong Y, Walsh MD, Cummings MC, Wright RG, Khoo SK, Parsons PG, McGuckin MA, Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours, J Pathol 183, 311–7 (1997).
McGuckin MA, Walsh MD, Hohn BG, Ward BG, Wright RG, Prognostic significance of MUC1 epithelial mucin expression in breast cancer, Hum Pathol 26, 432–9 (1995).
Beum PV, Singh J, Burdick M, Hollingsworth MA, Cheng PW, Expression of core 2 beta-1,6-N-acetylglucosaminyltransferase in a human pancreatic cancer cell line results in altered expression of MUC1 tumor-associated epitopes, J Biol Chem 274, 24641–8 (1999).
Ligtenberg MJ, Buijs F, Vos HL, Hilkens J, Suppression of cellular aggregation by high levels of episialin, Cancer Res 52, 2318–24 (1992).
Wesseling J, van der, Valk, Sw, Hilkens J, A mechanism for inhibition of E-cadherin-mediated cell–cell adhesion by the membrane-associated mucin episialin/MUC1, Molecular Biology of the Cell 7, 565–77 (1996).
Wesseling J, van, der, Valk, Sw, Vos HL, Sonnenberg A, Hilkens J, Episialin (MUC1) overexpression inhibits integrinmediated cell adhesion to extracellular matrix components, J Cell Biol 129, 255–65 (1995).
Suwa T, Hinoda Y, Makiguchi Y, Takahashi T, Itoh F, Adachi M, Hareyama M, Imai K, Increased invasiveness of MUC1 and cDNA-transfected human gastric cancer MKN74 cells, Int J Cancer 76, 377–82 (1998).
Baeckstrom D, Hansson GC, Nilsson O, Johansson C, Gendler SJ, Lindholm L, Purification and characterization of a membranebound and a secreted mucin-type glycoprotein carrying the carcinoma-associated sialyl-Lea epitope on distinct core proteins, J Biol Chem 266, 21537–47 (1991).
Baeckstrom D, Nilsson O, Price MR, Lindholm L, Hansson GC, Discrimination of MUC1 mucins from other sialyl-Le(a)-carrying glycoproteins produced by colon carcinoma cells using a novel monoclonal antibody, Cancer Res 53, 755–61 (1993).
Baeckstrom D, Karlsson N, Hansson GC, Purification and characterization of sialyl-Le(a)-carrying mucins of human bile; evidence for the presence of MUC1 and MUC3 apoproteins, J Biol Chem 269, 14430–7 (1994).
Hanski C, Drechsler K, Hanisch FG, Sheehan J, Manske M, Ogorek D, Klussmann E, Hanski ML, Blank M, Xing PX, et al., Altered glycosylation of the MUC-1 protein core contributes to the colon carcinoma-associated increase of mucin-bound sialyl-Lewis(x) expression, Cancer Res 53, 4082–8 (1993).
Hanski C, Hanski ML, Zimmer T, Ogorek D, Devine P, Riecken EO, Characterization of the major sialyl-Lex-positive mucins present in colon, colon carcinoma, and sera of patients with colorectal cancer, Cancer Res 55, 928–33 (1995).
Ho JJ, Siddiki B, Kim YS, Association of sialyl-Lewis(a) and sialyl-Lewis(x) with MUC-1 apomucin in a pancreatic cancer cell line, Cancer Res 55, 3659–63 (1995).
Regimbald LH, Pilarski LM, Longenecker BM, Reddish MA, Zimmermann G, Hugh JC, The breast mucin MUC1 as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer, Cancer Res 56, 4244–9 (1996).
Denda-Nagai K, Fujita K, Fujime M, Nakatsugawa S, Ishigaki T, Irimura T, Absence of correlation of MUC1 expression to malignant behavior of renal carcinoma in experimental systems, Clin Exp Metastasis 18, 77–81 (2000).
Spicer AP, Rowse GJ, Lidner TK, Gendler SJ, Delayed mammary tumor progression in Muc-1 null mice, J Biol Chem 270, 30093–101 (1995).
Makiguchi Y, Hinoda Y, Imai K, Effect of MUC1 mucin, an anti-adhesion molecule, on tumor cell growth, Jpn J Cancer Res 87, 505–11 (1996).
Irimura T, McIsaac AM, Carlson DA, Yagita M, Grimm EA, Menter DG, Ota DM, Clary KR, Soluble factor in normal tissues that stimulates high-molecular-weight sialoglycoprotein production by human colon carcinoma cells, Cancer Res 50, 3331–8 (1990).
Zhang K, Sikut R, Hansson GC, A MUC1 mucin secreted from a colon carcinoma cell line inhibits target cell lysis by natural killer cells, Cell Immunol 176, 158–65 (1997).
van de Wiel-van Kemenade E, Ligtenberg MJ, de Boer AJ, Buijs F, Vos HL, Melief CJ, Hilkens J, Figdor CG, Episialin (MUC1) inhibits cytotoxic lymphocyte-target cell interaction, J Immunol 151, 767–76 (1993).
Zhang K, Baeckstrom D, Brevinge H, Hansson GC, Secreted MUC1 mucins lacking their cytoplasmic part and carrying sialyl-Lewis a and x epitopes from a tumor cell line and sera of colon carcinoma patients can inhibit HL-60 leukocyte adhesion to E-selectin-expressing endothelial cells, Journal of Cellular Biochemistry 60, 538–49 (1996).
Agrawal B, Krantz MJ, Reddish MA, Longenecker BM, Cancerassociated MUC1 mucin inhibits human T-cell proliferation, which is reversible by IL-2, Nat Med 4, 43–9 (1998).
Paul S, Bizouarne N, Paul A, Price MR, Hansson GC, Kieny MP, Acres RB, Lack of evidence for an immunosuppressive role for MUC1, Cancer Immunol Immunother 48, 22–8 (1999).
Pemberton L, Taylor PJ, Gendler SJ, Antibodies to the cytoplasmic domain of the MUC1 mucin show conservation throughout mammals, Biochem Biophys Res Commun 185, 167–75 (1992).
Pandey P, Kharbanda S, Kufe D, Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein, Cancer Res 55, 4000–3 (1995).
Zrihan LS, Baruch A, Elroy SO, Keydar I, Wreschner DH, Tyrosine phosphorylation of the MUC1 breast cancer membrane proteins, Cytokine receptor-like molecules, Febs Letters 356, 130–6 (1994).
Yamamoto M, Bharti A, Li Y, Kufe D, Interaction of the DF3/MUC1 breast carcinoma-associated antigen and betacatenin in cell adhesion, J Biol Chem 272, 12492–4 (1997).
Baruch A, Hartmann M, Zrihan-Licht S, Greenstein S, Burstein M, Keydar I, Weiss M, Smorodinsky N, Wreschner DH, Preferential expression of novel MUC1 tumor antigen isoforms in human epithelial tumors and their tumor-potentiating function, Int J Cancer 71, 741–9 (1997).
Baruch A, Hartmann M, Yoeli M, Adereth Y, Greenstein S, Stadler Y, Skornik Y, Zaretsky J, Smorodinsky NI, Keydar I, Wreschner DH, The breast cancer-associated MUC1 gene generates both a receptor and its cognate binding protein, Cancer Res 59, 1552–61 (1999).
Takahashi T, Makiguchi Y, Hinoda Y, Kakiuchi H, Nakagawa N, Imai K, Yachi A, Expression of MUC1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient, J Immunol 153, 2102–9 (1994).
Magarian-Blander J, Ciborowski P, Hsia S, Watkins SC, Finn OJ, Intercellular and intracellular events following the MHCunrestricted TCR recognition of a tumor-specific peptide epitope on the epithelial antigen MUC1, J Immunol 160, 3111–20 (1998).
Burchell J, Taylor PJ, Boshell M, Gendler S, Duhig T, A short sequence, within the amino acid tandem repeat of a cancerassociated mucin, contains immunodominant epitopes, Int J Cancer 44, 691–6 (1989).
Fontenot JD, Mariappan SV, Catasti P, Domenech N, Finn OJ, Gupta G, Structure of a tumor associated antigen containing a tandemly repeated immunodominant epitope, J Biomol Struct Dyn 13, 245–60 (1995).
Hinoda Y, Takahashi T, Hayashi T, Suwa T, Makiguchi Y, Itoh F, Adachi M, Imai K, Enhancement of reactivity of anti-MUC1 core protein antibody and killing activity of anti-MUC1 cytotoxic T cells by deglycosylation of target tissues or cells, J Gastroenterol 33, 164–71 (1998).
Noto H, Takahashi T, Makiguchi Y, Hayashi T, Hinoda Y, Imai K, Cytotoxic T lymphocytes derived from bone marrowmononuclear cells of multiple myeloma patients recognize an underglycosylated form of MUC1 mucin, Int Immunol 9, 791–8 (1997).
Agrawal B, Reddish MA, Longenecker BM, In vitro induction of MUC-1 peptide-specific type 1 T lymphocyte and cytotoxic T lymphocyte responses from healthy multiparous donors, J Immunol 157, 2089–95 (1996).
Apostolopoulos V, Loveland BE, Pietersz GA, McKenzie IF, CTL in mice immunized with human mucin 1 are MHC-restricted, J Immunol 155, 5089–94.
Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ, Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients, Cancer Res 54, 2856–60 (1994).
Rughetti A, Turchi V, Ghetti CA, Scambia G, Panici PB, Roncucci G, Mancuso S, Frati L, Nuti M, Human B-cell immune response to the polymorphic epithelial mucin, Cancer Res 53, 2457–9 (1993).
Kamoshida S, Tsutsumi Y, Expression of MUC-1 glycoprotein in plasma cells, follicular dendritic cells, myofibroblasts and perineurial cells: immunohistochemical analysis using three monoclonal antibodies, Pathol Int 48, 776–85 (1998).
Agrawal B, Krantz MJ, Parker J, Longenecker BM, Expression of MUC1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation, Cancer Res 58, 4079–81 (1998).
Ding L, Lalani EN, Reddish M, Koganty R, Wong T, Samuel J, Yacyshyn MB, Meikle A, Fung PY, Taylor PJ, et al., Immunogenicity of synthetic peptides related to the core peptide sequence encoded by the human MUC1 mucin gene: effect of immunization on the growth of murine mammary adenocarcinoma cells transfected with the human MUC1 gene, Cancer Immunol Immunother 36, 9–17 (1993).
Apostolopoulos V, Xing PX, McKenzie IF, Murine immune response to cells transfected with human MUC1: immunization with cellular and synthetic antigens, Cancer Res 54, 5186–93 (1994).
Karanikas V, Hwang LA, Pearson J, Ong CS, Apostolopoulos V, Vaughan H, Xing PX, Jamieson G, Pietersz G, Tait B, Broadbent R, Thynne G, McKenzie IFC, Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein, J Clin Invest 100, 2783–92 (1997).
Goydos JS, Elder E, Whiteside TL, Finn OJ, Lotze MT, A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma, Journal of Surgical Research 63, 298–304 (1996).
Sandrin MS, Vaughan HA, Xing PX, McKenzie IF, Natural human anti-Gal alpha(1,3)Gal antibodies react with human mucin peptides, Glycoconj J 14, 97–105 (1997).
Apostolopoulos V, Osinski C, McKenzie IF, MUC1 crossreactive Gal alpha(1,3)Gal antibodies in humans switch immune responses from cellular to humoral, Nat Med 4, 315–20 (1998).
Peat N, Gendler SJ, Lalani N, Duhig T, Taylor PJ, Tissue-specific expression of a human polymorphic epithelial mucin (MUC1) in transgenic mice, Cancer Res 52, 1954–60 (1992).
Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ, Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model, Cancer Res 58, 315–21 (1998).
Carr-Brendel V, Markovic D, Ferrer K, Smith M, Taylor-Papadimitriou J, Cohen EP, Immunity to murine breast cancer cells modified to express MUC-1, a human breast cancer antigen, in transgenic mice tolerant to human MUC-1, Cancer Res 60, 2435–43 (2000).
Acres B, Apostolopoulos V, Balloul JM, Wreschner D, Xing PX, Ali-Hadji D, Bizouarne N, Kieny MP, McKenzie IF, MUC1-specific immune responses in human MUC1 transgenic mice immunized with various human MUC1 vaccines, Cancer Immunol Immunother 48, 588–94 (2000).
>Lees CJ, Apostolopoulos V, Acres B, Ramshaw I, Ramsay A, Ong CS, McKenzie IF, Immunotherapy with mannan-MUC1 and IL-12 in MUC1 transgenic mice, Vaccine 19, 158–62 (2000).
Gong J, Chen D, Kashiwaba M, Kufe D, Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells, Nat Med 3, 558–61 (1997).
Gong J, Chen D, Kashiwaba M, Li Y, Chen L, Takeuchi H, Qu H, Rowse GJ, Gendler SJ, Kufe D, Reversal of tolerance to human MUC1 antigen in MUC1 transgenic mice immunized with fusions of dendritic and carcinoma cells, Proc Natl Acad Sci USA 95, 6279–83 (1998).
Hiltbold EM, Ciborowski P, Finn OJ, Naturally processed class II epitope from the tumor antigen MUC1 primes human CD4+ T cells, Cancer Res 58, 5066–70 (1998).
Hiltbold EM, Vlad AM, Ciborowski P, Watkins SC, Finn OJ, The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells, J Immunol 165, 3730–41 (2000).
Hiltbold EM, Alter MD, Ciborowski P, Finn OJ, Presentation of MUC1 tumor antigen by class I MHC and CTL function correlate with the glycosylation state of the protein taken up by dendritic cells, Cell Immunol 194, 143–9 (1999).
von Mensdorff-Pouilly S, Petrakou E, Kenemans P, van Uffelen K, Verstraeten AA, Snijdewint FG, van Kamp GJ, Schol DJ, Reis CA, Price MR, Livingston PO, Hilgers J, Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and n-acetylgalactosamine (GalNAc) peptides, Int J Cancer 86, 702–12 (2000).
Bohm CM, Mulder MC, Zennadi R, Notter M, Schmitt-Graff A, Finn OJ, Taylor-Papadimitriou J, Stein H, Clausen H, Riecken EO, Hanski C, Carbohydrate recognition on MUC1-expressing targets enhances cytotoxicity of a T cell subpopulation, Scand J Immunol 46, 27–34 (1997).
Pietersz GA, Li W, Osinski C, Apostolopoulos V, McKenzie IF, Definition of MHC-restricted CTL epitopes from non-variable number of tandem repeat sequence of MUC1, Vaccine 18, 2059–71 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Denda-Nagai, K., Irimura, T. MUC1 in carcinoma-host interactions. Glycoconj J 17, 649–658 (2000). https://doi.org/10.1023/A:1011039013134
Issue Date:
DOI: https://doi.org/10.1023/A:1011039013134