Abstract
Purpose. Gene therapy has been limited by the immunogenicity of viral vectors, by the inefficiency of cationic liposomes, and by the rapid degradation in vivofollowing the injection of naked DNA. The present work describes a new approach that enables the non-invasive, non-viral gene therapy of the brain and peripheral organs following an intravenous injection.
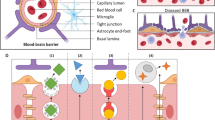
Methods. The plasmid DNA encoding β-galactosidase is packaged in the interior of neutral liposomes, which are stabilized for in vivo use by surface conjugation with polyethyleglycol (PEG). The tips of about 1% of the PEG strands are attached to a targeting monoclonal antibody (MAb), which acts as a “molecular Trojan Horse” to ferry the liposome carrying the gene across the biological barriers of the brain and other organs. The MAb targets the transferrin receptor, which is enriched at both the blood-brain barrier (BBB), and in peripheral tissues, such as liver and spleen.
Results. Expression of the exogenous gene in brain, liver, and spleen was demonstrated with β-galactosidase histochemistry, which showed persistence of gene expression for at least 6 days after a single intravenous injection of the pegylated immunoliposomes. The persistence of the transgene was confirmed by Southern blot analysis.
Conclusions. Widespread expression of an exogenous gene in brain and peripheral tissues is induced with a single intravenous administration of plasmid DNA packaged in the interior of pegylated im- munoliposomes. The liposomes are formulated to target specific receptor systems that enable receptor-mediated endocytosis of the complex into cells in vivo. This approach allows for non-invasive, non-viral gene therapy of the brain.
Similar content being viewed by others
REFERENCES
A. H. Hamawy, L. Y. Lee, R. G. Crystal, and T. K. Rosengart. Cardiac angiogenesis and gene therapy: A strategy for myocardial revascularization. Curr. Opinion in Cardiology. 14:515-522 (1999).
G. R. Akkaraju, J. Huard, E. P. Hoffman, W. F. Goins, R. Pruchnic, S. C. Watkins, J. B. Cohens, and J. C. Glorioso. Herpes simplex virus vector-mediated dystrophin gene transfer and expression in MDX mouse skeletal muscle. J. Gene Med. 1:280-289 (1999).
Y. Liu, D. Liggitt, W. Zhong, G. Tu, K. Gaensler, and R. Debs. Cationic liposome-mediated intravenous gene delivery. J. Biol. Chem. 270:24864-24870. (1995).
C. J. Wheeler, P. L. Flegner, Y. J. Tsai, J. Marshall, L. Sukhu, S. G. Doh, J. Hartikka, J. Nietupski, M. Manthorpe, M. Nichols, M. Plewe, X. Liang, J. Norman, A. Smith, and S.H. Cheng. A novel cationic lipid greatly enhances plasmid DNA delivery and expression in mouse lung. Proc. Natl. Acad. Sci. USA 93:11454-11459 (1996).
C. H. Wu, J. M. Wilson, and G. Y. Wu. Targeting genes: delivery and persistent expression of a foreign gene driven by mammalian regulatory elements in vivo. J. Biol. Chem. 264:16985-16987 (1989).
M. Cotton, F. Langle-Rouault, H. Kirlappos, E. Wagner, K. Metchtler, M. Zenke, H. Beug, and M. L. Birnstiel. Transferrin-polycation-mediated introduction of DNA into human leukemic cells: stimulation by agents that affect the survival of transfected DNA or module transferrin receptor levels. Proc. Natl. Acad. Sci. USA 87:4033-4037 (1990).
T. Ferkol, J.C. Perales, F. Mularo, and R.W. Hanson. Receptor-mediated gene transfer into macrophages. Proc. Natl. Acad. Sci. USA 93:101-105 (1996).
M. E. Barry, D. Pinto-Gonzalez, F. M. Orson, G. J. McKenzie, G. R. Petry, and M. A. Barry. Role of endogenous endonucleases and tissue site in transfection and CpG-mediated immune activation after naked DNA injection. Hum. Gene Ther. 10:2461-2480 (1999).
N. Shi and W. M. Pardridge. Non-invasive gene targeting to the brain. Proc. Natl. Acad. Sci. USA 97:7567-7572 (2000).
P. A. Monnard, T. Oberholzer, and P. Luisi. Entrapment of nucleic acids in liposomes. Biochim. Biophys. Acta. 1329:39-50 (1997).
D. Papahadjopoulos, T. M. Allen, A. Gabizon, E. Mayhew, K. Matthay, S. K. Huang, K. D. Lee, M. C. Woodle, D. D. Lasic, C. Redemann, et al. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA 88:11460-11464 (1991).
J. Huwyler, D. Wu, and W.M. Pardridge. Brain drug delivery of small molecules using immunoliposomes. Proc. Natl. Acad. Sci. USA 93:14164-14169 (1996).
K. A. Basclain and G. P. Jeffrey. Coincident increase in periportal expression of iron proteins in the iron-loaded rat liver. J. Gastroenterol. Hepatol. 14:659-668 (1999).
M. J. Coloma, H. J. Lee, A. Kurihara, E. M. Landaw, R. J. Boado, S. L. Morrison, and W. M. Pardridge. Transport across the primate blood-brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm. Res. 17:266-274 (2000).
C. Plank, M. X. Tang, A. R. Wolfe, and F. C. Szoka. Branched cationic peptides for gene delivery: role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum. Gene Ther. 10:319-332 (1999).
H. E. J. Hofland, D. Nagy, J.J. Liu, K. Spratt, Y. L. Lee, O. Danos, and S. M. Sullivan. In vivo gene transfer by intravenous administration of stable cationic lipid/DNA complex. Pharm. Res. 14:742-749 (1997).
J. Huwyler, J. Yang, and W. M. Pardridge. Targeted delivery of daunomycin using immunoliposomes: pharmacokinetics and tissue distribution in the rat. J. Pharmacol. Exp. Ther. 282:1541-1546 (1997).
J. Huwyler and W. M. Pardridge. Examination of blood-brain barrier transferrin receptor by confocal fluorescent microscopy of unfixed isolated rat brain capillaries. J. Neurochem. 70:883-886 (1998).
D. C. Mash, J. Pablo, D. D. Flynn, S. M. Efange, and W. J. Weiner. Characterization and distribution of transferrin receptors in the rat brain. J. Neurochem. 55:1972-1979 (1990).
Y. Zhang and W. M. Pardridge. Rapid transferrin efflux from brain to blood across the blood-brain barrier. J. Neurochem. 76: 1597-1600 (2001).
K. W. C. Mok, A. M. I. Lam, and P. R. Cullis. Stabilized plasmid-lipid particles: factors influencing plasmid entrapment and transfection properties. Biochim. Biophys. Acta. 1419:137-150 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shi, N., Boado, R.J. & Pardridge, W.M. Receptor-Mediated Gene Targeting to Tissues In Vivo Following Intravenous Administration of Pegylated Immunoliposomes. Pharm Res 18, 1091–1095 (2001). https://doi.org/10.1023/A:1010910523202
Issue Date:
DOI: https://doi.org/10.1023/A:1010910523202