Abstract
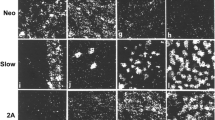
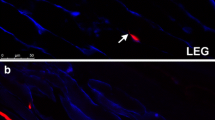
Because complex structural differences in adult extraocular muscles may have physiological and pathophysiological significance, the three-dimensional pattern of myosin heavy chain (MHC) isoform expression within the orbital and global layers of the muscle bellies compared with the distal tendon ends was quantitatively assessed. Three of the six extraocular muscles of adult rabbits were examined for immunohistologic expression of all fast, fast IIA/X, slow, neonatal and developmental MHC isoforms. The percentages of myofibers positive for each of these 5 myosin isoforms were determined in the orbital and global layers. There were relatively similar patterns of fast and slow MHC expression in the orbital and global layers of each of the three muscles examined. There were high levels of developmental MHC in the orbital layers, but significantly fewer developmental MHC positive myofibers in the global layer. The most variable expression was found with the neonatal MHC. There were significant differences between the longitudinal expression of the various isoforms in the middle of each muscle compared with the tendon end. In the orbital layer of all three muscles examined, the large numbers of fibers positive for fast MHC in the middle of the muscle dramatically decreased at the tendon end, with a concomitant increase in expression of slow myosin. There was a greater number of developmental MHC-positive myofibers at the tendon end than in the middle of the muscle in all three muscles examined. In the global layer, the IIA/X-positive myofibers comprised only half of the total number of fast-positive myofibers whereas in the orbital layer they comprised all or almost all of the fast positive myofibers. The configuration of the extraocular muscles is more complex than might be indicated by previous studies. The lateral rectus muscle had the most individual pattern of MHC expression when compared with the inferior rectus and inferior oblique muscles. Together with dramatic cross-sectional MHC fiber type differences between the orbital and global layers of the muscles, there are pronounced longitudinal differences in the proportions of myofibers expressing these five MHC isoforms in the middle region of the muscles and those in the distal tendon ends. This longitudinal progression appears to occur both within single myofibers, as well as within the series of myofibers that comprise the length of the muscle. We also confirm that the number of myofibers is reduced at the tendonous end while the cross-sectional area of each of the remaining myofibers is proportionally increased with regard to those in the muscle belly. Future studies may yet require two additional schemes for anatomic classification of the named extraocular muscles. One will be based on immunohistochemical features of their constituent myofibers as a supplement to classifications based on their electron microscopic appearance, innervation patterns or relative position with regard to the globe and orbit. Another will be based on the proportional length and longitudinal position of individual myofibers within an individual extraocular muscle.
Similar content being viewed by others
References
Alway SE (1993) Stretch induces non-uniform isomyosin expression in the quail anterior latissimus dorsi muscle. Anat Rec 237: 1-7.
Bottinelli R, Betto R, Schiaffino S and Reggiani C (1994) Maximum shortening velocity and coexistence of myosin heavy chain isoforms in single skinned fast fibres of rat skeletal muscle. J Muscle Res Cell Motil 15: 413-419.
Bredman JJ, Weijs WA, Korfage HAM, Brugman R and Moorman AFM (1992) Myosin heavy chain expression in rabbit masseter muscle during postnatal development. J Anat 180: 263-274.
Brueckner JK, Itkis O and Porter JD (1996) Spatial and temporal patterns of myosin heavy chain expression in developing rat extraocular muscle. J Muscle Res Cell Motil 17: 297-312.
Butler-Browne GS, Eriksson PO, Laurent C and Thornell LE (1988) Adult human masseter muscle fibers express myosin isozymes characteristic of development. Muscle and Nerve 11: 610-620.
Camoretti-Mercado B, Qin Y, Jakovcic S, Salazar-Grueso S and Zak R (1996) Developmental shift of myosin heavy chain mRNA expression due to neural factor(s) and muscle activity. Am J Physiol 271: C1350-C1357.
Chanaud CM, Prattt CA and Loeb GE (1991) Functionally complex muscles of the cat hindlimb. V. The roles of histochemical fiber-type regionalization and mechanical heterogeneity in differential muscle activation. Exp Brain Res 85: 300-313.
Chiarandini DJ and Davidowitz J (1979) Structure and function of extraocular muscle fibers. In: Zadunaisky JA and Davson H (eds.) Current Topics in Eye Research. (pp. 91-142) Academic Press, New York.
Close RI and Luff AR (1974) Dynamic properties of inferior rectus of the rat. J Physiol 236: 259-270.
D'Albis A, Couteaux R, Goubel F, Janmot C and Mira JC (1995) Response to denervation of rabbit soleus and gastrocnemius muscles. Time course study of postnatal changes in myosin isoforms, fiber types, and contractile properties. Biol Cell 85: 9-20.
Davidowitz J, Philips G and Breinin GM (1977) Organization of the orbital surface layer in rabbit superior rectus. Invest Ophthalmol Vis Sci 16: 711-729.
Denardi C, Ausoni S, Moretti P, Gorza L, Velleca M, Buckingham M and Schiaffino S (1993) Type 2X-myosin heavy chain is coded by a muscle fiber type-specific and developmentally regulated gene. J Cell Biol 123: 823-835.
de Ruiter CJ, Habets PEMH, de Haan A and Sargeant AJ (1996) In vivo IIX and IIB fiber recruitment is compartment related. J Appl Physiol 81: 933-942.
Essig DA, Devol DL, Bechtel PJ and Trannel TJ (1991) Expression of embryonic myosin heavy chain mRNA in stretched adult chicken skeletal muscle. Am J Physiol 260: C1325-C1331.
Frueh BR, Hayes A, Lynch GS and Williams DA (1994) Contractile properties and temperature sensitivity of the extraocular muscles, the levator and superior rectus, of the rabbit. J Physiol 475: 327-336.
Galler S, Schmitt TL and Pette D (1994) Stretch activation, unloaded shortening velocity, and myosin heavy chain isoforms of rat skeletal muscle fibres. J Physiol 478: 513-521.
Gao L and Kennedy JM (1992) Repression of the embryonic myosin heavy chain phenotype in regenerating chicken slow muscle is dependent on innervation. Muscle and Nerve 15: 419-429.
Gaunt AS and Gans C (1992) Serially arranged myofibers: an unappreciated variant in muscle architecture. Experientia 48: 864-868.
Gauthier GF (1990) Differential distribution of myosin isoforms among the myofibrils of individual developing muscle fibers. J Cell Biol 110: 693-701.
Goldberg SJ and Shall MS (1997) Lateral rectus whole muscle and motor unit contractile measures with the extraocular muscles intact. J Neurosci Meth 78: 47-50.
Goldberg SJ, Wilson KE and Shall MS (1997) Summation of extraocular motor unit tensions in the lateral rectus muscle of the cat. Muscle and Nerve 20: 1229-1235.
Gurahian SM and Goldberg SJ (1987) Fatigue of lateral rectus and retractor bulbi motor units in cat. Brain Res 415: 281-292.
Hermanson JW (1997) Architecture and the division of labor in the extensor carpi radialis muscle of horses. Acta Anat 159: 127-135.
Hess A (1961) The structure of slow and fast extrafusal muscle fibers in the extraocular muscles and their nerve endings in guinea pigs. J Cell Comp Physiol 58: 63-80.
Izumo S, Nadal-Ginard B and Mahdavi V (1986) All members of the MHC multigene family respond to thyroid hormones in a highly tissue specific manner. Science 231: 597-600.
Jacoby J and Ko K (1993) Sarcoplasmic reticulum fast Ca2.-pump and myosin heavy chain expression in extraocular muscles. Invest Ophthalmol Visc Sci 34: 2848-2858.
Jacoby J, Ko K, Weiss C and Rushbrook JI (1989) Systematic variation in myosin expression along extraocular muscle fibers of the adult rat. J Muscle Res Cell Motil 11: 25-40.
Johnson BD, Wilson LE, Zhan WZ, Watchko JF, Daood MJ and Sieck GC (1994) Contractile properties of the developing dia-phragm correlate with myosin heavy chain phenotype. J Appl Physiol 77: 481-487.
Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G and Saltin B (1990) Aging alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand 140: 55-62.
Kwa SHS, Weijs WA and Juch PJW (1995) Contraction characteristics and myosin heavy chain composition of rabbit masseter motor units. J Neurophysiol 73: 538-549.
Lander T, Wirtschafter JD and McLoon LK (1996) Orbicularis oculi muscle fibers are relatively short and heterogeneous in length. Invest Ophthalmol Visc Sci 37: 1732-1739.
Loeb GE, Pratt CA, Chanaud CM and Richmond FJR (1987) Distribution and innervation of short, interdigitated muscle fibers in parallel-fibered muscles of the cat hindlimb. J Morphol 191: 1-15.
Loughna PT, Izumo S, Goldspink G and Nadal-Ginard B (1990) Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development 109: 217-223.
Lynch GS, Frueh BR and Williams DA (1994) Contractile properties of single skinned fibers from the extraocular muscles, the levator and superior rectus, of the rabbit. J Physiol 475: 337-346.
Mayr R, Gottschall J, Gruber H and Neuhuber W (1975) Inter-nal structure of cat extraocular muscle. Anat Embryol 148: 25-34.
McKoy G, Leger ME, Bacou F and Goldspink G (1998) Differential expression of myosin heavy chain isoforms in four functionally diverse rabbit skeletal muscles during pre-and postnatal develop-ment. Devel Dyn 211: 193-203.
McLoon LK and Wirtschafter JD (1996) N-CAM is expressed in mature extraocular muscles in a pattern conserved among three species. Invest Ophthalmol Visc Sci 37: 318-327.
Meredith MA and Goldberg SJ (1986) Contractile differences between muscle units in the medial rectus and lateral rectus muscles in the cat. J Neurophysiol 6: 50-62.
Naumann K and Pette D (1994) Effects of chronic stimulation with different impulse patterns on the expression of myosin isoforms in rat myotube cultures. Differentiation 55: 203-211.
Oishi Y, Ishihara A, Yamamoto H and Miyamoto E (1998) Hindlimb suspension induces the expression of multiple myosin heavy chain isoforms in single fibres of the rat soleus muscle. Acta Physiol Scand 162: 127-134.
Peachey L (1971) The structure of the extraocular muscle fibers of mammals. In: Bach-Y-Rita P and Collins C (eds.) The Control of Eye Movements. (pp.47-66) Academic Press, New York.
Pedrosa-Domellof F, Eriksson PO, Butler-Browne GS and Thornell LE (1992) Expression of alpha-cardiac myosin heavy chain in mammalian skeletal muscle. Experientia 48: 491-494.
Peuker H and Pette D (1997) Quantitative analyses of myosin heavy-chain mRNA and protein isoforms in single fibers reveal a pronounced fiber heterogeneity in normal rabbit muscles. Eur J Biochem 247: 30-36.
Rushbrook JI, Weiss C and Yao TT (1991) Developmental myosin heavy chain progression in avian type IIB muscle fibers. J Muscle Res Cell Motil 12: 281-291.
Rushbrook JI, Weiss C, Ko K, Feuerman MH, Carleton S, Ing A and Jacoby J (1994) Identification of alpha-cardiac myosin heavy chain mRNA and protein in extraocular muscle of the adult rabbit. J Muscle Res Cell Motil 15: 505-515.
Sakuma K, Yamaguchi A, Ohmori K and Katsuta S (1995) Non-uniform changes in fiber types in the soleus muscle of the developing rat. Eur J Appl Physiol 70: 132-137.
Sant'Ana Pereira JAA, Wessels A, Nijtmans L, Moorman AFM and Sargeant AJ (1995) New method for the accurate characterization of single human skeletal muscle fibers demonstrates a relation between mATPase and MyHC expression in pure and hybrid fiber types. J Muscle Res Cell Motil 16: 21-34.
Sawchak JA, Lewis S and Shafiq SA (1989) Coexpression of myosin isoforms in muscle of patients with neurogenic disease. Muscle and Nerve 12: 679-689.
Schiaffino S and Reggiani C (1994) Myosin isoforms in mammalian skeletal muscle. J Appl Physiol 77: 493-501.
Sola OM, Kakulas BA and Thomas R (1991) Anatomy of the latissimus dorsi muscle II. Segmental anatomy and function. In: Carpentier A, Chaques JC and Grandjean P (eds.) Cardiomyopla-sty. (pp. 68-71) Futura, New York.
Spencer RF and Porter JD (1988) Structural organization of the extraocular muscles. In: Buttner-Ennever JA (ed.) Neuroanatomy of the Oculomotor System. (pp. 33-79) Elsevier, New York.
Stal P, Eriksson PO, Schiaffino S, Butler-Browne GS and Thornell LE (1994) Differences in myosin composition between oro-facial, masticatory and limb muscles: enzyme-, immunohisto-, and biochemical studies. J Muscle Res Cell Motil 15: 517-534.
Wieczorek DR, Periasamay M, Butler-Browne GS, Whalen R and Nadal-Ginard B (1985) Co-expression of multiple myosin heavy chain genes, in addition to a tissue specific one, in extraocular musculature. J Cell Biol 101: 618-629.
Yang H, Alnaqeeb M, Simpson H and Goldspink G (1997) Changes in muscle fiber type, muscle mass and IGF-I gene expression in rabbit skeletal muscle subjected to stretch. J Anat 190: 613-622.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McLoon, L.K., Rios, L. & Wirtschafter, J.D. Complex three-dimensional patterns of myosin isoform expression: differences between and within specific extraocular muscles. J Muscle Res Cell Motil 20, 771–783 (1999). https://doi.org/10.1023/A:1005656312518
Issue Date:
DOI: https://doi.org/10.1023/A:1005656312518