Abstract
Clonidine is a partial agonist at brain α2-adrenoceptors (α2AR), but also has high affinity (KD = 51 nM) in homogenate binding assays for non-adrenergic imidazoline-binding sites (I-sites; imidazoline receptors). Herein, an autoradiographic comparison of [3H]-clonidine binding to I-sites and α2AR in sections of human brain is reported. For I-sites, the adrenergic component of 50 nM [3H]-clonidine binding was masked with either 60 μM norepinephrine (NE; α2AR agonist) or 12.5 μM methoxy-idazoxan (MIDX; selective α2AR antagonist), whereas the remaining non-adrenergic sites were studied by displacement with 20 μM cirazoline. Levels of [3H]-clonidine binding to α2AR and I-sites, determined in adjacent tissue sections, were positively correlated across 27 brain regions (p = 0.0003; r2 = 0.385). The principal olivary nucleus and the rostral portion of the ventrolateral medulla had highest ratios of I-sites: α2AR (>4:1). Quantitative transepts drawn across hippocampal images revealed α2AR enrichments in the CA-1 and inner molecular layers of the dentate gyrus—areas not enriched in I-sites. Competition curves were generated for I-sites in caudate sections using 10 ligands known to distinguish between I1 and I2 subtypes. The rank-order of affinities were cirazoline > harmane > BDF6143 >> idazoxan = tizanidine (affinities of agmatine, efaroxan, moxonidine, NE, and oxymetazoline were too low to be reliable). Only the endogenous I-site ligand, harmane, had a monophasic displacement curve at the non-adrenergic sites (Ki = 521 ± 12 nM). In conclusion: 1) the distribution of non-adrenergic [3H]-clonidine binding sites in human brain sections was correlated with, but distinct from α2AR; and 2) the affinities of these sites was distinct from α1AR, α2AR, I1 or I2 sites as previously defined in membrane binding assays. The properties of this non-adrenergic [3H]-clonidine binding site are consistent with I-sites previously labeled by [3H]-cirazoline in rat brain.
Similar content being viewed by others
Main
Clonidine is the prototypic imidazoline compound that can elicit a diversity of CNS effects (Buccafusco 1992). Originally thought to be a selective agonist for α2-adrenoceptors (α2AR), clonidine is now realized to possesses only slightly lower affinity for non-adrenergic imidazoline receptors (IR) compared to α2AR (Bousquet et al. 1984; Ernsberger et al. 1987).
The first subtype of IR (IR1) was discovered by comparing the hypotensive effects of clonidine to other non-imidazoline α2AR agonists, which were far less hypotensive agents than clonidine when micro-injected into the rostral ventrolateral medulla (RVLM) of anesthetized rats (Bousquet et al. 1984). In those studies, the pharmacological control of blood pressure was found to best correlate with agents classified as imidazoline compounds (Ernsberger and Haxhiu 1997). In fact, the recommended therapeutic doses for clonidine and other imidazoline compounds to treat patients with hypertension have been positively correlated with their affinities for non-adrenergic imidazoline binding sites (of the I1 subtype)—whereas no such correlation exists with their affinities for α2AR (Buccafusco et al. 1995).
In psychiatry, novel imidazoline compounds are currently being explored for possible neuro-protective properties (Regunathan et al. 1999), for treating drug withdrawal symptoms (Vandergriff et al. 1999), and as possible antidepressants (Raddatz and Lanier 1997). Second-generation imidazoline anti-hypertensives are also now available, which include rilmenidine and moxonidine (Molderings 1997). Rilmenidine and moxonidine possess approximately 5-fold and 100-fold selective affinities, respectively, for I1 over α2AR sites (Ernsberger et al. 1995a; Piletz et al. 1996), and this affinity difference probably explains their diminished sedative side-effects relative to clonidine (van Zwieten 1997).
Like most receptor families, there are several IR subtypes identified using radioligand binding techniques. I1 sites have high affinity and I2 sites have low affinity for clonidine (Michel and Ernsberger 1992). By contrast, planar imidazoline compounds, like idazoxan, exhibit preferential affinity for I2 sites, of which there are two subclasses: I2A and I2B (Eglen et al. 1998). There are at least two other I-site subtypes defined by various other imidazolines: a cirazoline-sensitive I-site in kidney and brain (Angel et al. 1995) and a novel efaroxan-sensitive I-site in pancreas (Monks et al. 1999). In addition, the search for endogenous ligands has identified two putative IR neurotransmitters: a decarboxylated metabolite of arginine, agmatine (Reis and Regunathan 1999), and a β-carboline, harmane (Hudson et al. 1999).
Only one previous autoradiographic study exists of [3H]-clonidine binding to brain I1 sites (De Vos et al. 1994). While improving on that autoradiographic method, we have now provided a more detailed visualization of those sites in human brain. A comparison is made of the distribution of non-adrenergic versus adrenergic sites labeled by [3H]-clonidine across 27 brain regions. De Vos et al. (1994) did not previously distinguish non-adrenergic versus adrenergic sites labeled by [3H]-clonidine. Special attention is given to the hippocampus, where previous studies of depressed suicide victims have previously revealed a decrease in IR immunoreactivity by western blotting (Piletz et al. 2000b). A pharmacological rank-ordering of affinities is also presented for I-sites labeled by [3H]-clonidine using competitive ligands known to distinguish I1, I2, and α2AR sites (taken from previous homogenate binding studies).
In summary, we are describing a non-α2AR binding site for [3H]-clonidine which has a distribution distinct from [3H]-clonidine-labeled α2AR in human brain, and the pharmacology of this site resembles an imidazoline binding site previously thought by De Vos and coworkers (1994) to be an I1 site.
MATERIALS AND METHODS
Human Tissue Collection
Discarded brain tissues from five subjects (three males and two females) who died of sudden natural or accidental causes were collected at the time of autopsy at the Cuyahoga County Coroner's Office, Ohio, in accordance with an approved Institutional Review Board protocol. Tissues were coded to protect the identity of subjects. Deaths occurred within four hours of injury. Causes of death were determined by the county medical examiner. Four of the subjects died of cardiovascular failure and one died of asphyxiation secondary to smoke inhalation. The average age of the subjects was 52 ± 10 yr (age range = 24 to 80 yr). All cadavers were refrigerated prior to autopsy, immediately upon arrival at the coroner's facility. The average postmortem interval (from death to freezing of brain tissue) was 16 ± 3 h (range 6 to 21 h). The coroner performed toxicological screens for 50 compounds in the blood, bile, urine, and/or vitreous fluids of cadavers to identify numerous psychoactive and other compounds. Four of the subjects had no detectable drugs in the toxicological screen. One subject had evidence of lidocaine in the blood. Postmortem autopsies revealed no record of neurological or psychiatric histories for any of the subjects.
At autopsy, blocks of brain tissue were dissected. The techniques for dissection of blocks of tissue have been published for frontal cortex (Brodmann's area 10) and hippocampus (Klimek et al. 1999), and for locus coeruleus (Klimek et al. 1997). The tissue block containing the hypothalamus was dissected just anterior to the substantia nigra and was sectioned in the coronal plane. A block of tissue containing the medulla was obtained by a transverse cut just caudal to the caudal end of the locus coeruleus, and this block of tissue was cut in the transverse plane, perpendicular to the rostro-caudal axis of the medulla. Tissue blocks were dipped in 2-methylbutane cooled on dry ice (−50°C), placed on powdered dry ice for 10 min, and then stored in an ultra-cold freezer (−82 °C) until sectioned. Frozen blocks of brain tissue were mounted on a specimen chuck of a cryostat microtome (Cryocut 1800, Leica, Reichert-Jung, Deerfield, IL) and tissue sections (20 μm thick) were cut at −16°C and thaw-mounted onto gelatin-coated microscope slides. Sections were dried under refrigeration and stored at −82°C until assayed.
Quantitative Autoradiography
Quantitative autoradiography of [3H]-clonidine binding was performed using a modification of the method of De Vos et. al. (1994). [3H]-clonidine (50 nM) was used to label both α2AR and I-sites in adjacent tissue sections. Sections were removed from the freezer and dried on the counter top at room temperature (22°C). Subsequently, sections were incubated with [3H]-clonidine, in the presence and absence of competitors as designated below, in a reaction buffer containing 50 mM Tris-HCl, 2 mM MgCl2, and 252 μM ascorbic acid (pH 7.4). This was performed by placing a small drop of reaction buffer containing the radioligand, directly on each slide-mounted section, and then incubating 1 h in a humidity chamber at 22°C. Unbound radioligand was removed by three consecutive washes (1 min each) of the slides in ice-cold reaction buffer, then dipped for 1 sec in ice cold water to remove buffer salts. Slide-mounted sections were dried immediately afterwards under a stream of air at room temperature overnight. Slides were apposed, along with brain-mash calibrated 3H standards (American Radiolabeled Chemicals Inc., St. Louis, Mo.), to Hyperfilm-3H (Amersham, Arlington Hts, IL) in x-ray cassettes and stored at room temperature for four weeks. Films were processed with GBX developer and fixer (Eastman Kodak Co., Rochester, NY). The same sections were then stained with cresyl violet to identify anatomical structures. The resulting autoradiograms were quantified using the M2 Microcomputer Imaging Device (Imaging Research, St. Catharines, Ontario, Canada). Hippocampal fields (Klimek et al. 1999) were outlined based on morphological criteria (Duvernoy 1988). Brainstem and medullary regions were identified with the aid of an atlas (Paxinos and Huang 1995).
Total binding was determined by incubating sections with 50 nM [3H]-clonidine without any competitors. In adjacent sections, binding to α2AR was determined by subtracting the binding of [3H]-clonidine in the presence of 60 μM norepinephrine (NE) from the total binding of [3H]-clonidine. Binding to I-sites was determined by subtracting the binding of [3H]-clonidine in the presence of 60 μM NE plus 20 μM cirazoline from the binding of [3H]-clonidine in the presence of 60 μM NE. NE exhibited negligible binding to I-sites under the conditions of this assay (Figure 5). On the other hand, cirazoline is known to displace [125I]p-iodoclonidine from human I1 sites (Ki = 99 nM) (Piletz et al. 1996). The percentage of total binding that was specific binding (including I-sites and α2AR) was 72 ± 1 % (means ± S.E.M. of all brain regions from all subjects). Of this specific binding, 54 ± 1% was binding to I-sites and 46 ± 1% was binding to α2AR.
Inhibition of the binding of [3H]-clonidine (50 nM) to I-sites in consecutive human caudate section wipes by imidazoline and non-imidazoline compounds. In all cases, the binding of [3H]-clonidine to α2-adrenoceptors was prevented by adding 12.5 μM methoxy-idazoxan to the binding reaction mixture. Competing ligands were (▾) agmatine, (⋄) NE, (▪) moxonidine, (○) oxymetazoline, (▴) BDF6143, (▿) idazoxan, (♦) tizanidine, (□) harmane, and (•) cirazoline. Each point represents the mean ± S.E.M. of values generated from three separate experiments
Competition curves were generated from slide wipes using ligand concentrations from 10−10 to 10−4 M (11 points in triplicate for each of 3–4 subjects). The measurements were made on serial adjacent caudate sections (2–4 sections per slide, wiped together). After incubation for 1 h and a rapid cold water dip, the caudate sections were wiped from the slides onto S&S #32 glass fiber filters (Schleicher and Schuell, Keene, NH). Radioactivity was measured by liquid scintillation counting. In a typical experiment, total radioactivity remaining in the presence of 60 μM NE was 1503 ± 222 cpm, and that remaining in the presence of 20 μM cirazoline plus 60 μM NE was 433 ± 28 cpm. Competition curves were analyzed by both LIGAND (McPherson 1985) and Prism (GraphPad, San Diego, CA), producing similar results. Data were modeled to one-site and two-site fits (McPherson 1985) and the preferred model was chosen by an F test. IC50 values were computed from 2-3 experiments and were expressed as mean ± SEM.
Drugs
[3H]-clonidine (66.5 Ci/mmol) was purchased from NENTM (Life Science Products, Inc., Boston, MA) and stored at −20°C before reconstitution in reaction buffer (defined below). Moxonidine and BDF6143 were gifts of Lilly Forschung GmbH Company (Hamburg, Germany). Tizanidine was a gift of Sandoz Pharmaceuticals (Tokyo, Japan). Efaroxan was a gift from Pierre Fabre Pharmacie (Castres Cedex, France). Cirazoline and RX821002 (MIDX; methoxy-idazoxan) were purchased from Research Biochemicals International (Natick, MA). Agmatine, harmane, NE, oxymetazoline, and idazoxan (IDX) were from Sigma Chemical Company (St. Louis, MO). All chemicals were of the highest grade available. Drug solutions were made fresh daily before each experiment. Drug stock solutions were prepared (0.01 M) in either distilled water or 10 mM acetic acid depending on their solubilities and were serially diluted in the reaction buffer.
Technical Note
Several differences are noteworthy comparing our method to the autoradiographic method of De Vos and coworkers (1994): 1) The concentration of [3H]-clonidine in our study (50 nM) was nearer its KD concentration for I-sites (57 nM), rather than 8 nM used by De Vos and coworkers; 2) MIDX (12.5 μM, instead of 2 μM used by De Vos and coworkers) and NE (60 μM) were compared as displacing agents to demonstrate that the adrenergic binding of [3H]-clonidine was of the α2AR type, not α1AR, as well as to ensure complete masking of α2AR in the case of I-sites; 3) In our study, non-specific binding was assessed with 20 μM cirazoline rather than with 10 μM cirazoline, because we found 20 μM cirazoline resulted in more complete inhibition of [3H]-clonidine binding (Figure 5); 4) Binding reactions were conducted for 60 min, as compared to 20 min by De Vos and colleagues (1994). This was based on earlier binding studies (Piletz et al. 1991); and 5) De Vos and colleagues used the antagonist, [3H]-MIDX, to assess α2AR binding. By contrast, we used the agonist [3H]-clonidine to assess I-sites and α2AR binding.
RESULTS
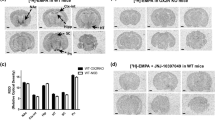
The specific binding of [3H]-clonidine to α2AR and I-sites was quantified by autoradiography in 27 human brain regions, including specific areas of prefrontal cortex, hippocampus, caudate, hypothalamus, pons, and the medulla oblongata (Table 1) . Comparison of [3H]-clonidine binding to α2AR and I-sites reveals overlapping, yet distinctly different distributions. For example, at the level of the medulla, there was considerable similarity between the distribution of I-sites and α2AR (Figure 1). In fact, there was a significant linear correlation between the amounts of [3H]-clonidine binding to α2AR and to I-sites when considering all brain regions in a scatter plot (p = .0003, r2 = 0.385; Figure 2).
Autoradiograms of the binding of 50 nM [3H]-clonidine to I-sites (left) and to α2-adrenoceptors (right) in human medulla. Left panel: the specific binding of [3H]-clonidine to I-sites generated by digital subtraction of an autoradiogram of [3H]-clonidine binding in the presence of NE (60 μM) plus cirazoline (20 μM) from an autoradiogram of the binding of [3H]-clonidine in the presence of NE alone. Right panel: the specific binding of [3H]-clonidine to α2-adrenoceptors generated by digital subtraction of the autoradiogram of the binding of [3H]-clonidine in the presence of NE (60 μM) from the autoradiogram of the binding of [3H]-clonidine alone. For both panels, the two autoradiograms used to produce the image were generated from adjacent tissue sections. The calibration bar illustrates the relationship between the color spectrum and radioligand binding density. The arrows indicate the rostral ventrolateral region, based on human brain atlas maps
Correlation between the binding of [3H]-clonidine to I-sites and to α2-adrenoceptors in 27 brain regions from human prefrontal cortex, hippocampus, caudate, hypothalamus, pontine region, and the medulla oblongata (p = .0003, r2 = 0.385). Each point represents the mean value of binding obtained for each region (five subjects/region)
Despite the overall correlation between the densities of α2AR and I-sites (Figure 2), there were distinct sub-regional differences in the distributions of α2AR and I-sites in the brain. Highest binding to I-sites was in the principle nucleus of the inferior olive (130 ± 18 fmol/mg protein) wherein the amount of binding was 10-fold higher than that in the white matter of the prefrontal cortex (Table 1, Figure 2). Other areas of high density for [3H]-clonidine binding to I-sites included the polymorphic layer of the hippocampus, dorsal and median raphe, dentate gyrus, hypothalamus, locus coeruleus, central gray, and the hypoglossal nucleus (Table 1). Lowest [3H]-clonidine binding to I-sites was in the white matter of the prefrontal cortex, the striosomes of the caudate, and the pyramidal tract of the medulla. The distribution of I-sites contrasted the distribution of [3H]-clonidine binding to α2AR. The highest binding to α2AR occurred in the molecular layer of CA1 of the hippocampus and in the hypothalamus. Moderate amounts of binding were observed in the locus coeruleus.
To further illustrate the distinction between α2AR and I-sites, quantitative transepts were drawn through specific binding images of hippocampi using an image analysis system (Figure 3). Amounts of binding across a transept through a subregion may differ from the levels outlined in the entire subregion (i.e., data in Table 1). In the outlining method (Table 1), uneven binding densities within subregions are averaged by the computer program. In the transept method (Figure 3), pixel by pixel densities are recorded across the subregions. Therefore, quantitative transepts should allow a closer examination of complex regions, like the hippocampus, where uneven binding distributions exist. Histograms of the transept densities of [3H]-clonidine binding to I-sites and α2AR are shown in Figures 3 and 4 . The difference between these sites was most obvious in the molecular layer of CA1. There, binding to α2AR clearly exceeded that of I-sites by 4:1, whereas in other subregions of the hippocampus the ratios of α2AR:I sites were closer to 1:1.
Comparison of the distribution of the binding of [3H]-clonidine to I-sites (A) and to α2-adrenoceptors (B) in the human hippocampus using norepinephrine (NE) to identify α2-adrenoceptors. (A): The specific binding of [3H]-clonidine to I-sites was generated by digital subtraction of an autoradiogram of [3H]-clonidine binding in the presence of NE (60 μM) plus cirazoline (20 μM) from an autoradiogram of the binding of [3H]-clonidine in the presence of NE alone. (B): The specific binding of [3H]-clonidine to α2-adrenoceptors generated by digital subtraction of the autoradiogram of the binding of [3H]-clonidine in the presence of NE (60 μM) from the autoradiogram of the binding of [3H]-clonidine alone. For both images, the two autoradiograms used to produce the image shown were generated from adjacent tissue sections. White outline bars overlaid upon the images of A and B indicate where a quantitative transept bar was placed. (C): The quantitative distributions of [3H]-clonidine binding to I-sites (lower line) and to α2-adrenoceptors (upper line in 4), expressed pixel by pixel along the horizontal axis. Representative regions along these transepts are indicated by numbers: 1 = CA3; 2 = CA4; 3 = polymorphic area; 4 = Molecular layer of CA1; 5 = transitional area between CA1 and subiculum
Comparison of the distribution of the binding of [3H]-clonidine to I-sites (A) and to α2-adrenoceptors (B) in the human hippocampus using methoxy-iodazoxan (MIDX) to identify α2-adrenoceptors. (A): The specific binding of [3H]-clonidine to I-sites was generated by digital subtraction of an autoradiogram of [3H]-clonidine binding in the presence of MIDX (12.5 μM) and cirazoline (20 μM) from an autoradiogram of the binding of [3H]-clonidine in the presence of MIDX alone. (B): The specific binding of [3H]-clonidine to α2-adrenoceptors generated by digital subtraction of the autoradiogram of the binding of [3H]-clonidine in the presence of MIDX (12.5 μM) from the autoradiogram of the binding of [3H]-clonidine alone. For both images, the two autoradiograms used to produce the image shown were generated from adjacent tissue sections. White outline bars overlaid upon the images of A and B indicate where a quantitative transept bar was placed. (C): The quantitative distributions of [3H]-clonidine binding to I-sites (lower line) and to α2-adrenoceptors (upper line), expressed pixel by pixel along the horizontal axis. Representative regions along these transepts are indicated by numbers: 1 = CA3; 2 = CA4; 3 = polymorphic layer; 4 = Molecular layer of CA1; 5 = transitional area between CA1 and subiculum
To rule out the possibility that the agonist-induced state of α2AR might in some way account for brain I-sites (e.g., an artifact of using the agonists, [3H]-clonidine and NE), we defined α2AR with either NE (60 μM) or the α2AR antagonist, MIDX (12.5 μM). MIDX is a selective antagonist for α2AR with very low affinity for the human brain I-sites (IC50 at I-sites ⩾ 60 μM) (De Vos et al. 1994; Flamez et al. 1997). We observed that specific binding to α2AR was only slightly greater using MIDX, and the specific binding to I-sites was only slightly lower using MIDX compared to the results with NE. Furthermore, the relative distributions of binding to α2AR and I-sites were identical using either of these competitors (Figure 4, Panel C). The near identity of these results with either displacing agent makes it highly unlikely that an α2AR affinity state could account for I-sites.
Competition curves were next generated for [3H]-clonidine binding (under a mask of 12.5 μM MIDX) at caudate I-sites (Figure 5). [3H]-Clonidine was found to have a KD = 57 ± 3 nM for I-sites, in keeping with the results of De Vos and colleagues (1994). The head of the caudate was chosen for these studies because of the availability of tissue. Five compounds were capable of completely displacing [3H]-clonidine from caudate I-sites, but these mostly had shallow competition curves (Figure 5). The only exception was the β-carboline, harmane, which exhibited a monophasic competition curve (Figure 5). The rank order of IC50 values for I-sites (generated using a single site model) was as follows: cirazoline > harmane > BDF6143 > idazoxan = tizanidine. Agmatine, efaroxan, moxonidine, NE, and oxymetazoline did not inhibit [3H]-clonidine binding sufficiently to determine IC50 values. Actually, agmatine failed to displace any I-sites labeled by [3H]-clonidine in caudate Figure 5, (Table 2).
For comparative purposes, Table 2 shows our Ki values beside those previously determined by De Vos and colleagues (1994), who also used the head of the human caudate for competition binding studies. The rank-order for human I1 sites in homogenate binding assays is known to be moxonidine ⩾ oxymetazoline > tizanidine ⩾ agmatine ⩾ efaroxan = clonidine > cirazoline > BDF6143 > idazoxan >> NE (Piletz et al. 1996). The rank-order for human I2 sites in homogenate binding assays is cirazoline > idazoxan > oxymetzoline >> moxonidine ⩾ efaroxan > clonidine (Piletz and Sletten 1993). Thus, the I-site(s) under study has(have) a unique pharmacological profile.
Moxonidine, a compound with preferential I1 affinity, was found to have low affinity in caudate sections for I-sites labeled by [3H]-clonidine (Figure 5, Table 2). To examine this further, moxonidine was used in parallel with cirazoline to define non-adrenergic binding of [3H]-clonidine in a number of regions of the medulla from three subjects. The RVLM is often cited as mediating the non-adrenergic hypotensive effects of clonidine, moxonidine, and rilmenidine (Chan et al. 1996). Table 3 shows four conditions of [3H]-clonidine binding: (a) without any competitors (total binding); (b) in the presence of 60 μM NE (displacing α2AR); (c) in the presence of 60 μM NE plus 20 μM cirazoline (displacing α2AR and I-sites); and (d) in the presence of 60 μM NE plus 5 μM moxonidine (displacing α2AR and only a small additional amount of I-sites). The amount of [3H]-clonidine binding in any region remaining in the presence of NE was not significantly different from the amount remaining in the presence of NE plus moxonidine, but was significantly greater than the amount of binding remaining in the presence of NE plus cirazoline (Table 3). Even in the RVLM (Figure 1, arrow), [3H]-clonidine binding was not substantially more displaced by moxonidine than NE (Table 3).
DISCUSSION
In the present study, the distributions of [3H]-clonidine binding to I-sites and α2AR were distinct (Figures 1, 3, and 4) but correlated across 27 brain regions (Figure 2). As expected, the RVLM (Figure 1, arrow) had a high ratio of I-sites: α2AR (nearly 5:1) compared to other brain regions (Table 1). This is noteworthy because the RVLM is the site of the anti-hypertensive action ascribed to IR1 stimulation when imidazolines are micro-injected into the brainstem (Bousquet et al. 1984). However, a number of brain regions were more abundantly enriched with I-sites than the RVLM, notably the inferior olive, pontine regions, and hypothalamus (Table 1). Furthermore, our competition binding curves (Figure 5) make it doubtful that the non-adrenergic site labeled by [3H]-clonidine in the human RVLM is of the I1 subtype (see below).
A clear distinction in the regional distribution of [3H]-clonidine labeling to α2AR and I-sites was obvious in the hippocampus (see Figures 3 and 4). The distribution of I-sites and α2AR was virtually identical whether defined by masking [3H]-clonidine binding with an α2AR agonist (NE) or an α2AR antagonist (MIDX; Figures 3 and 4). Approximately 50% of the [3H]-clonidine binding in the human hippocampal CA1 molecular layer was to α2AR. This compared to ≈35% binding to I-sites and ≈15% non-specific binding in the same CA1 region.
It should be noted that α2AR would be nearly saturated at a concentration of 50 nM [3H]-clonidine, since clonidine has a high affinity at each of the human α2AR (α2AAR, KD = 1.5 nM; α2BAR, KD = 7.8 nM; α2CAR, KD = 1.3 nM for [125I]p-iodoclonidine) (Piletz et al. 1996). On the other hand, I-sites would only be half-saturated (KD = 55 nM for [125I]p-iodoclonidine). Therefore, the proportions of these two sites in, for instance, the CA1 molecular layer might actually be re-estimated to be 6:5 for I-sites:α2AR. Using similar calculations, the CA4 region of the hippocampus might be estimated to possess a 9:3 ratio of I-sites:α2AR as labeled by [3H]-clonidine. Thus, I-sites and α2AR appeared unevenly distributed in the hippocampus with each site having a unique distribution.
A variety of previous autoradiographic studies of I-sites, mostly in animals, exist in the literature (De Vos et al. 1994; Ernsberger et al. 1994; King et al. 1995; Lione et al. 1998; Mallard et al. 1992). Unfortunately, the results of those previous studies have been as diverse as the radioligands used to define them. Besides us, only De Vos and colleagues (1994) have used [3H]-clonidine to visualize brain I-sites. Our results compare favorably in a number of ways with theirs (Table 2). On the other hand, Ernsberger and coworkers (1994) reported diffuse [125I]p-iodoclonidine binding to “I1-like” sites in rat brainstem sections. Their diffuse images contrasted sharply with the highly regionalized images of Figures 1, 3, and 4. For instance, the striking intensity of I-sites in the olivary nuclei (Figure 1) appears to be a “signature” for human I-sites labeled with [3H]-clonidine, but this was not reported for [125I]p-iodoclonidine autoradiograms (Ernsberger et al. 1994). We believe this discrepancy from the results with [125I]p-iodoclonidine reported by Ernsberger and coworkers (1994) may be related to the lipophilicity of [125I]p-iodoclonidine (see below). There are also autoradiographic images from rat brain using [3H]idazoxan (Mallard et al. 1992), [3H]rilmenidine (King et al. 1995), and [3H]2-(2-benzofuranyl-2-imidazoline (Lione et al. 1998). The latter three studies have all related their findings to I2 sites (King et al. 1995; Lione et al. 1998; Mallard et al. 1992).
By convention, I-sites labeled with [3H]-clonidine have been designated as I1 sites (Ernsberger et al. 1987). However, De Vos and colleagues (1994) used cautionary language when making an assignment of their non-adrenergic [3H]-clonidine binding site as an I1 subtype. They reported that idazoxan and guanoxan had higher affinities than expected for an I1 site (Table 2), but clonidine and rilmenidine had affinities consistent with an I1 subtype. In that same study, they clearly distinguished their site from the I2 site labeled by [3H]-idazoxan and from the α2AR labeled by [3H]-MIDX (De Vos et al. 1994). Therefore, De Vos and colleagues concluded: “At first glance, IR appear to correspond to the I1 imidazoline receptor subtype … however, this comparison is subject to some caution.” Their cautionary note is echoed by our present results.
Special emphasis may be given to our choice of cirazoline for defining specific binding to I-sites (Figure 5). NE or MIDX were used to displace [3H]-clonidine from adrenergic sites, and cirazoline was used (under NE or MIDX masks) to displace [3H]-clonidine from non-adrenergic I-sites. Nearly identical images were obtained with either NE or MIDX as displacers (Figures 3 and 4). Therefore, any hypothesis invoking an agonist-induced state of [3H]-clonidine binding to α2AR in order to explain the existence of I-sites seems unlikely. Equally important, cirazoline is an imidazoline α1AR agonist without high affinity for known human α2AR subtypes (Ki values for α2AR subtypes vary from 193 to 657 nM) (Piletz et al. 1996). At 50 nM, [3H]-clonidine would bind minimally to α1AR because the Ki value of clonidine for brain α1AR is 750 nM (Glossmann et al. 1981).
[3H]-cirazoline has previously been shown to label a non-adrenergic/ non-I1 / non-I2 site in brain homogenate binding assays (Angel et al. 1995). In characterizing that I-site, Angel et al. (1995) reported the Ki values of several of the same drugs studied here. They reported a rank ordering of affinities for I-sites of cirazoline (Ki = 4 nM) > clonidine (Ki = 50 nM) > idazoxan (Ki = 158 nM) >> oxymetazoline (22,000 nM) > efaroxan (219,000 nM) > (-) epinephrine (635,000 nM). This rank-order of Ki values corresponds closer to our data (Table 2) than any other I-sites reported. Therefore, the same receptor(s) may have been under study by [3H]-clonidine displacement with cirazoline (in our study) and by [3H]-cirazoline displacement with clonidine (Angel et al. 1995). The identity and function of the [3H]-cirazoline-sensitive I-site studied by Angel and coworkers (1995) remains unknown.
De Vos and colleagues (1994) found no correlation between I-sites labeled with [3H]-clonidine and α2AR sites labeled with [3H]-MIDX (r2 = − 0.068) when comparing the densities of these two sites in human brain regions. This is a surprising difference from the positive correlation we observed (p = .0003, r2 = 0.385) across 27 brain regions between these sites (Figure 2). However, of the eight brain regions in common between our two studies (cortical layers 1, 2–4, 5–6, hippocampal layer CA1, dentate gyrus, caudate putamen, raphe nuclei, and olivary nucleus), we also found no correlation between I-sites labeled with [3H]-clonidine and α2AR sites labeled with [3H]-clonidine (p = .58, r2 = 0.053). Therefore, the discrepancy between our studies could simply be due to different brain regions under study. It should be emphasized that De Vos and coworkers (1994) used the selective α2AR-antagonist, [3H]-MIDX, to measure α2AR binding, while we used the mixed α2AR/I-site agonist [3H]-clonidine.
De Vos and coworkers (1994) did not report on α2AR binding with [3H]-clonidine, although they used it for I-sites. Agonists, like [3H]-clonidine, preferentially induce a high affinity state of the α2AR (Alburges et al. 1993), whereas antagonists bind equally to low and high affinity states of α2AR. This difference in the states of α2AR labeled by [3H]-clonidine (high affinity states), versus both low and high affinity states in their study (De Vos et al. 1994) might underlie the different correlations between our studies. For instance, the correlation observed in Figure 2 may in fact relate to G-proteins in common with α2AR and I-sites.
The subtype or subtypes of I-sites labeled by [3H]-clonidine in human brain remains open to question. In competition studies with human caudate sections, we observed the affinities of I-sites labeled by [3H]-clonidine were not consistent with either an I1 or I2 site (Table 1). Three potent I1 ligands (efaroxan, oxymetazoline, and moxonidine) did not displace [3H]-clonidine binding from caudate I-sites, even at relatively high concentrations (Table 1). The possibility that the I-sites labeled by [3H]-clonidine are actually I2 sites also is unlikely because higher than expected affinities were observed for several agents (i.e., BDF6143 and rilmenidine affinities were 4-fold and 54-fold higher, respectively, than expected for human I2 sites) (Table 2) (Diamant et al. 1992). Furthermore, [3H]-clonidine binding was examined in human medullary slices with the goal of identifying a typical I1 site (i.e., moxonidine-displaceable). A series of medullary slices that included the human RVLM were studied (Figure 1, arrow). Our autoradiographic technique was the same as in caudate, except, in addition to the presence of 20 μM cirazoline in some slices, 5 μM moxonidine was added to measure the non-specific binding in adjacent sections. Yet, moxonidine competed for only slightly more total sites than NE in the medullary slices (Table 3). Oxymetazoline was also ineffective at displacing [3H]-clonidine from I-sites in the medulla (data not shown). Moxonidine was also found to be an ineffective displacer of I-sites in the hippocampus and prefrontal cortex (data not shown). Thus, we found no autoradiographic evidence for typical I1 sites labeled by [3H]-clonidine, even in the medulla.
It should also be mentioned that the conditions in our autoradiographic procedure, while routine for many receptors (Klimek et al. 1997), were vastly different from those recommended for filtration binding assays of homogenate preparations of I1 sites (Ernsberger et al. 1995b). Optimal buffer conditions for homogenate I1 sites invoke low osmotic and ionic strengths (Ernsberger et al. 1995b). Furthermore, purified plasma membranes are considered useful to lower the signal-to-noise ratio of I1 sites in most filtration binding assays of I1 sites (Ernsberger et al. 1995b). Also, a high flow-through vacuum system is considered critical for filtration binding assays of I1 sites, due to their fast off-rate of binding (Piletz et al. 1991). Hypo-osmolarity and hypotonic buffer conditions could not be used autoradiographically, because these conditions destroy the anatomical integrity of slide-mounted tissue sections. Hence, comparisons of affinities derived from autoradiographic binding studies and homogenate binding studies may not be legitimate for I1 or I2 sites. It remains a possibility that the different conditions used in the present study might have significantly altered the affinities of certain ligands, such as moxonidine, for true I1-sites.
Mention should also be made that most previous membrane binding studies of I1 and α2AR sites (Piletz and Sletten 1993; Klimek et al. 1999) have used [125I]-p-iodoclonidine as radioligand. In preliminary studies, we found that if [125I]-p-iodoclonidine was substituted for [3H]-clonidine using the autoradiographic method, a substantial amount of [125I]-p-iodoclonidine binding was localized to white matter (data not shown). Although [3H]-para-aminoclonidine and [125I]-p-iodoclonidine are known to have similar properties (Alburges et al. 1993), we found little or no specific labeling of white matter with [3H]-clonidine. Therefore, even closely related imidazoline radioligands, like [3H]-clonidine and [125I]-p-iodoclonidine, might bind different sites.
The molecular basis of imidazoline receptors is another area of intense research. There is little doubt that the diversity of ligand-defined subtypes will be matched by protein subtypes. A cDNA clone which encodes a peptide with I1-like properties has recently been described (Ivanov et al. 1998; Piletz et al. 2000a) and, surprisingly, the predicted protein has a novel amino acid sequence, different from known receptors. Therefore, it is uncertain if the cloned I1 site is actually a true receptor (Piletz et al. 2000a). I2 sites are somewhat better understood, and have been physically linked to monoamine oxidases (Tesson et al. 1995). In addition, there are at least two subtypes of I2 sites that exhibit biphasic binding curves (Eglen et al. 1998). An I3 site has been hypothesized to reside on the ion conducting pore component of the ATP-sensitive potassium channel (Monks et al. 1999). To these sites we now add another I-site labeled similarly by [3H]-cirazoline (Angel et al. 1995) and [3H]-clonidine (present study).
Harmane had high affinity (Ki = 521 nM) for I-sites labeled by [3H]-clonidine in the human caudate (Figure 2). This high affinity Ki value supports the candidacy of harmane as an endogenous I-site ligand (Hudson 1999). Harmane has been reported to have high affinity for I1 sites in filtration binding assays and to be active in modulating blood pressure in the CNS (Hudson 1999). The other endogenous I-site ligand, agmatine, had no affinity whatsoever for I-sites in the present study. Little is known about the regional distribution of harmane in the brain, and future studies will be required to determine if it co-localizes with putative IR sites.
In summary, the distribution of a novel I-site for [3H]-clonidine has been described in human brain. The pharmacology of this site is in agreement with a [3H]-clonidine binding site described by De Vos and coworkers (1994) and a [3H]-cirazoline site described by Angel and coworkers (1995). It remains to be discovered what the function of this site is, but given the plethora of actions previously ascribed to clonidine (Buccafusco 1992), it should be of interest to psychiatry. Currently, we are attempting to determine whether this is the same I-site that was reported previously to be decreased in the hippocampus of depressed suicide victims (Piletz et al. 2000b) and increased in the frontal cortex of depressed suicide victims (Garcia-Sevilla et al. 1996).
References
Alburges ME, Bylund DB, Pundt LL, Wamsley JK . (1993): α2 agonist binding sites in brain: [125I]para-iodoclonidine versus [3H]para-aminoclonidine. Brain Res Bull 32: 97–102
Angel I, Le Rouzic M, Pimoule C, Graham D, Arbilla S . (1995): [3H]cirazoline as a tool for the characterization of imidazoline sites. Ann N Y Acad Sci 763: 112–124
Bousquet P, Feldman J, Schwartz J . (1984): Central cardiovascular effects of alpha adrenergic drugs: Differences between catecholamines and imidazolines. J Pharmacol Exp Ther 230: 232–236
Buccafusco JJ . (1992): Neuropharmacologic and behavioral actions of clonidine: Interactions with central neurotransmitters. Int Rev Neurobiol 33: 55–107
Buccafusco JJ, Lapp CA, Westbrooks KL, Ernsberger P . (1995): Role of medullary I1-imidazoline and α2-adrenergic receptors in the antihypertensive responses evoked by central administration of clonidine analogs in conscious spontaneously hypertensive rats. J Pharmacol Exp Ther 273: 1162–1171
Chan CK, Sannajust F, Head GA . (1996): Role of imidazoline receptors in the cardiovascular actions of moxonidine, rilmenidine and clonidine in conscious rabbits. J Pharmacol Exp Ther 276: 411–420
De Vos H, Bricca G, DeKeyser J, DeBacker J, Bousquet P, Vauquelin G . (1994): Imidazoline receptors, non-adrenergic idazoxan binding sites, and α2 adrenoceptors in the human central nervous system. Neuroscience 59: 589–598
Diamant S, Eldar-Geva T, Atlas D . (1992): Imidazoline binding sites in human placenta: Evidence for heterogeneity and a search for physiological function. Br J Pharmacol 106: 101–108
Duvernoy H . (1988): The Human Hippocampus. An Atlas of Applied Anatomy. JF Bergmann, Munich.
Eglen RM, Hudson AL, Kendall DA, Nutt DJ, Morgan NG, Wilson VG, Dillon MP . (1998): ‘Seeing through a glass darkly’: Casting light on imidazoline ‘I’ sites. Trends Pharmacol Sci 19: 381–390
Ernsberger P, Graves ME, Graff LM, Zakieh N, Nguyen P, Collins LA, Westbrooks KL, Johnson GG . (1995a): I1-Imidazoline receptors: Definition, characterization, distribution and transmembrane signalling. Ann NY Acad Sci 763: 22–42
Ernsberger P, Haxhiu MA . (1997): The I1-imidazoline-binding site is a functional receptor mediating vasodepression via the ventral medulla. Am J Physiol 42: R1572–R1579
Ernsberger P, Haxhiu MA, Graff LM, Collins LA, Dreshaj I, Grove DL, Graves ME, Schafer SG, Christen MO . (1994): A novel mechanism of action for hypertension control: Moxonidine as a selective I1 imidazoline agonist. Cardiovasc Drugs Ther 8: 27–41
Ernsberger P, Meeley MP, Mann JJ, Reis DJ . (1987): Clonidine binds to imidazole binding sites as well as α2-adrenoceptors in the ventrolateral medulla. Eur J Pharmacol 134: 1–13
Ernsberger P, Piletz JE, Graff LM, Graves ME . (1995b): Optimization of radioligand binding assays for I1 imidazoline sites. Ann NY Acad Sci 763: 163–168
Flamez A, De Backer J-P, Czerwiec E, Ladure P, Vauquelin G . (1997): Pharmacological characterization of I1 and I2 imidazoline receptors in human striatum. Neurochem Int 30: 25–30
Garcia-Sevilla JA, Escriba PV, Sastre M, Walzer C, Busquets X, Jaquet G, Reis DJ, Guimon J . (1996): Immunodetection and quantitation of imidazoline receptor proteins in platelets of patients with major depression and in brains of suicide victims. Arch Gen Psychiatry 53: 803–810
Glossmann H, Lubbecke F, Bellemann P . (1981): [125I]-HEAT, a selective, high-affinity, high specific activity ligand for α1 adrenoceptors. Naunyn Schm Arch Pharmacol 318: 1–9
Hudson A, Price R, Tyacke RJ, Lalies MD, Parker CA, Nutt DJ . (1999): Harmane, norharmane and tetrahydro beta-carboline have high affinity for rat imidazoline binding sites. Br J Pharmacol 126: 2P
Ivanov TR, Jones JC, Dontenwill M, Bousquet P, Piletz JE . (1998): Characterization of a partial cDNA clone detected by imidazoline receptor-selective antisera. J Auton Nerv Syst 72: 98–110
King PR, Gundlach AL, Louis WJ . (1995): Quantitative autoradiographic localization in rat brain of α2-adrenergic and non-adrenergic I-receptor binding sites labeled by [3H]rilmenidine. Brain Res 675: 264–278
Klimek V, Rajkowska G, Luker SN, Dilley G, Meltzer HY, Overholser JC, Stockmeier CA, Ordway GA . (1999): Brain noradrenergic receptors in major depression and schizophrenia. Neuropsychopharmacology 21: 69–81
Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, Ordway GA . (1997): Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci 17: 8451–8458
Lione LA, Nutt DJ, Hudson AL . (1998): Characterisation and localization of [3H]2-(2-benzofuranyl)-2-imidazoline binding in rat brain: A selective ligand for imidazoline I2 receptors. Eur J Pharmacol 353: 123–135
Mallard NJ, Hudson AL, Nutt DJ . (1992): Characterization and autoradiographical localization of non-adrenoceptor idazoxan binding sites in the rat brain. Br J Pharmacol 106: 1019–1027
McPherson CA . (1985): Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods 14: 213–228
Michel MC, Ernsberger P . (1992): Keeping an eye on the I site: Imidazoline-preferring receptors. Trends Pharmacol Sci 13: 369–370
Molderings GJ . (1997): Imidazoline receptors: Basic knowledge, recent advances and future prospects for therapy. Drugs Future 22 (7)): 757–772
Monks LK, Cosgrove KE, Dunne MJ, Ramsden CA, Morgan NG, Chan SLF . (1999): Affinity isolation of imidazoline binding proteins from rat brain using 5-amino-efaroxan as a ligand. FEBS Lett 447: 61–64
Paxinos G, Huang X-F . (1995) Atlas of the Human Brainstem. Academic Press, San Diego, CA
Piletz JE, Andorn AC, Unnerstall JR, Halaris A . (1991): Binding of [3H]-p-aminoclonidine to α2-adrenoceptor states plus a non-adrenergic site on human platelet plasma membranes. Biochem Pharmacol 42: 569–584
Piletz JE, Ivanov TR, Sharp JD, Ernsberger P, Chang C-H, Pickard RT, Gold G, Roth B, Zhu H, Jones JC, Baldwin J, Reis DJ . (2000a): Imidazoline receptor antisera-selected (IRAS) cDNA: Cloning and characterization. DNA Cell Biol 19: 319–330
Piletz JE, Sletten K . (1993): Nonadrenergic imidazoline binding sites on human platelets. J Pharmacol Exp Ther 267: 1493–1502
Piletz JE, Zhu H, Chikkala DN . (1996): Comparison of ligand binding affinities at human I1 imidazoline binding sites and the high affinity state of α2 adrenoceptor subtypes. J Pharmacol Exp Ther 279: 694–702
Piletz JE, Zhu H, Ordway G, Stockmeier C, Dilly G, Reis D, Halaris A . (2000b): Imidazoline receptor proteins in the hippocampus of major depressives. Biol Psychiat 48 (9))
Raddatz R, Lanier SM . (1997): Relationship between imidazoline/guanidinium receptive sites and monoamine oxidase A and B. Neurochem Int 30: 109–117
Regunathan S, Feinstein DL, Reis DJ . (1999): Anti-proliferative and anti-inflammatory actions of imidazoline agents. Are imidazoline receptors involved? Ann N Y Acad Sci 881: 410–419
Reis DJ, Regunathan S . (1999): Agmatine: An endogenous ligand at imidazoline receptors is a novel neurotransmitter. Ann N Y Acad Sci 881: 65–80
Tesson F, Limon-Boulez I, Urban P, Puype M, Vandekerckhove J, Coupry I, Pompon D, Parini A . (1995): Localization of I2-imidazoline binding sites on monoamine oxidases. J Biol Chem 270: 9856–9861
van Zwieten PA . (1997): Central imidazoline (I1) receptors as targets of centrally acting antihypertensives: Moxonidine and rilmenidine. J Hypertens 15: 117–125
Vandergriff J, Kallman MJ, Rasmussen K . (2000): Moxonidine, a selective imidazoline-1 receptor agonist, suppresses the effects of ethanol withdrawal on the acoustic startle response in rats. Biol Psychiat 47: 874–879
Acknowledgements
We are grateful to Josh Farley (University of Mississippi Medical Center) and Jane Schenck (University of Mississippi Medical Center) for their technical assistance, and to Dr. Violetta Klimek for her valuable suggestions. The authors gratefully acknowledge the assistance of Dr. Craig Stockmeier (University of Mississippi Medical Center), Lisa Konick (Case Western Reserve University), and Dr. Balraj and staff at the Cuyahoga County Coroner's office, Cleveland, Ohio. This research was supported by NIH grants MH42859, MH46692, and MH45488.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Piletz, J., Ordway, G., Zhu, H. et al. Autoradiographic Comparison of [3H]-Clonidine Binding to Non-Adrenergic Sites and α2-Adrenergic Receptors in Human Brain. Neuropsychopharmacol 23, 697–708 (2000). https://doi.org/10.1016/S0893-133X(00)00166-4
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(00)00166-4
Keywords
This article is cited by
-
Antidepressant-like action of agmatine in the acute and sub-acute mouse models of depression: a receptor mechanism study
Metabolic Brain Disease (2018)
-
Neuroimaging of mirtazapine enantiomers in humans
Psychopharmacology (2008)