Abstract
Background and Aim
Dialysis patients are a high-risk population and have a reduced immune response to vaccination against SARS-CoV-2. The aim of this study was to assess the humoral response to homologous Gam-COVID-Vac (Sputnik V) and heterologous Sputnik V/mRNA-1273 (Moderna) vaccination in dialysis patients. The vaccination scheme depended on dose availability and the prioritization of risk populations as established by the Argentine Ministry of Health.
Methods
Previous COVID-19 infection was determined in symptomatic patients. Binding IgG antibodies against the spike (S) receptor-binding domain (RBD) of SARS-CoV-2 (anti-S-RBD) concentration was assessed between 3 and 16 weeks after the boost dose. Anti-S-RBD antibodies were quantified using the Abbott Diagnostics SARS-CoV-2 IgG II Quant chemiluminescent microparticle immunoassay (CMIA) on an Architect i2000 SR and an Alinity I analyzer (Abbott Diagnostics, Abbott Park, Illinois, USA). To standardize the results to WHO binding antibody units (BAU), a correction factor for Abbott arbitrary units (AU) was applied where 1 BAU/mL equals 0.142 AU, as previously established by Abbott with the WHO international standard NIBSC 20–136. Following the manufacturer’s recommendations, samples were considered reactive for anti-S-RBD when titers were above 50 AU/mL (7.2 BAU/mL). An 80% protective effect (PROT-80) against symptomatic SARS-CoV-2 infection was assumed when anti-S-RBD titers were 506 BAU/ml or higher. Charlson Comorbidity Index (CCI) score was classified as mild = 1–2, moderate = 3–4, and severe ≥ 5. Side effects were evaluated until day 7 by patients´ self-reported questionnaire.
Results
One hundred seven participants were enrolled [n = 84 homologous (SpV/SpV), nn 23 heterologous (SpV/Mod)]. Median (IQR) age was 64 (50–75) years old and 79 (73.8%) were male. Additionally, 19 (22.6%) of the SpV/SpV and 4 (17.4%) of the SpV/Mod group had a prior confirmed SARS-CoV-2 infection (p = 0.589). In the overall population, 103 patients reached seroconversion (96.3%). Anti-S-RBD IgG median titers (IQR) were higher in the heterologous [1222 (288–5680) BAU/mL] than in the homologous scheme [447 (100–1551) BAU/mL], p = 0.022. In a linear model adjusted for age, gender, days from first vaccination to boost dose and days from the boost dose to the anti-S-RBD IgG determination, previous SARS-COV-2 infection (B: 2062.2; CI95: 1231.8–2892.6; p < 0.001), and SpV/Mod vaccination scheme (B: 1294.6; CI95: 435.58–2147.6; p = 0.003) were independently associated with anti-S-RBD levels. Finally, a higher frequency of adverse effects was associated with the heterologous scheme, although they were well tolerated by all individuals.
Conclusions
The present study provides evidence that the homologous SpV/SpV and heterologous SpV/Mod schemes showed good efficacy and safety in patients on chronic dialysis. These results could be useful for designing future vaccination strategies, especially aimed at this risk group.
Graphical abstract

Similar content being viewed by others
Introduction
Patients on chronic dialysis are at increased risk for severe coronavirus infectious disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) than the general population, with reported mortality rates of up to 28.3% [1, 2]. This situation is mainly related to a considerably higher mean patient age (approximately 65 years old) and the frequent presence of comorbid conditions such as obesity, diabetes, high blood pressure, socioeconomic deprivation, and frailty that are linked to a more severe COVID-19 course. Fortunately, effective vaccination schemes against SARS-CoV-2 inducing reduction of both infection and the risk of severe COVID-19 have been rapidly developed [3,4,5,6]. Moreover, when facing first dose serious adverse effects or dose supply shortages, introduced heterologous vaccination schemes have shown promising results [7,8,9,10,11]. Nevertheless, clinical trials do not provide information about vaccine efficacy in dialysis populations, and possible differences in immunogenicity among SARS-CoV-2 vaccination schemes are poorly understood due to their novelty. Particularly, the homologous Gam-COVID-VAC (Sputnik V) and the heterologous vaccination scheme including Sputnik V as a prime dose and mRNA-1273 (Moderna) as a booster dose have been barely studied since Sputnik V is not approved in all countries, its implementation suffers geographic limitations, and its approval by the European Medicines Agency (EMA) and the World Health Organization (WHO) is still awaited. Nonetheless, the National Administration of Medicines, Food, and Medical Technology of Argentina (ANMAT) approved Sputnik V emergency use in late December 2020. Sputnik V approval was followed by approval of the ChAdOx1 nCoV-19 vaccine (AZD1222), Sinopharm COVID‐19 (BBIBP‐CorV) vaccine, mRAN-1273 COVID-19 (Moderna), and BNT162b2 (Pfizer). However, due to the second dose shortage of Sputnik V, mRNA-1273 (Moderna) was frequently administered as the booster dose.
In this scenario, the present study aimed to assess the humoral response to homologous Sputnik V and heterologous Sputnik V/Moderna vaccination in dialysis patients.
Materials and Methods
Study Design and Population
From March to October 2021, subjects who underwent hemodialysis at the Centro de Educación Médica e Investigaciones Clínicas “Norberto Quirno” (CEMIC), Buenos Aires, Argentina, were included in this prospective cohort study. Inclusion criteria were (i) having received Sputnik V prime immunization, (ii) having received a boost dose of either Sputnik V (SpV/SpV) or Moderna (SpV/Mod) vaccines within 18 weeks post-prime dose, and (iii) having presented for monitoring of humoral immune response 3 weeks after the boost dose. Previous COVID-19 infection was determined in symptomatic patients or in persons in contact with symptomatic patients. The vaccination scheme depended on dose availability and the prioritization of risk populations as established by the Argentine Ministry of Health.
Immunogenicity
Binding IgG antibodies against the spike (S) receptor-binding domain (RBD) of SARS-CoV-2 (anti-S-RBD) concentration was assessed between 3 and 16 weeks after the boost dose. Anti-S-RBD antibodies were quantified using the Abbott Diagnostics SARS-CoV-2 IgG II Quant chemiluminescent microparticle immunoassay (CMIA) on an Architect i2000 SR and an Alinity I analyzer (Abbott Diagnostics, Abbott Park, Illinois, USA). To standardize the results to WHO binding antibody units (BAU), a correction factor for Abbott arbitrary units (AU) was applied where 1 BAU/mL equals 0.142 AU, as previously established by Abbott with the WHO international standard NIBSC 20–136 [12]. Following the manufacturer’s recommendations, samples were considered reactive for anti-S-RBD when titers were above 50 AU/mL (7.2 BAU/mL). An 80% protective effect (PROT-80) against symptomatic SARS-CoV-2 infection was assumed when anti-S-RBD titers were 506 BAU/ml or higher [13]. Charlson Comorbidity Index (CCI) score was classified as mild = 1–2, moderate = 3–4, and severe ≥ 5 [14].
Side effects
All patients were invited to complete an online questionnaire to report all possible post-boost vaccination adverse events requiring medical assistance. The intensity of adverse effects was graded as mild, moderate, and severe depending on the interference with daily activities.
Statistical analysis
Descriptive statistics and univariate analyses were performed. The outcome variable was the anti-S-RBD titer at least 3 weeks after the boost dose. Differences in anti-S-RBD levels and PROT-80 according to demographic and clinical parameters were evaluated. Categorical variables were expressed as numbers (percentage) and analyzed using the Chi-square test or Fisher’s exact test. Student’s t-test and the Mann–Whitney U test were used to compare independent continuous variables, expressed as median (interquartile range, IQR). For related continuous variables, the Wilcoxon signed-rank test was applied. Factors associated with anti-S-RBD levels with a p < 0.2 in the univariate analysis were evaluated in a general linear model adjusted for age and sex. Likewise, multivariate logistic regression models were developed to identify factors associated with PROT-80. Adjusted odds ratios (AOR) with their corresponding 95% confidence intervals (CI95) were calculated. Statistical analyses were carried out using the SPSS statistical software package release 23.0 (IBM SPSS Inc., Chicago, IL, USA).
Results
Study population
A total of 107 subjects were included in the study, 84 (78.5%) received SpV/SpV, and the remaining 23 (21.5%) the SpV/Mod scheme. Seventy-nine (73.8%) participants were male, and the median (IQR) age was 64 (50–75) years old. Overall, median time intervals were 91 (77–116) days from prime to boost dose (ΔP-B) and 32 (24–47) days from the boost dose to the anti-S-RBD IgG serological determination (ΔB-antiSRBD). Eighty-four (78.5%) individuals were naïve to SARS-CoV-2 infection at the time of prime vaccination, while 19 (22.6%) of those who received the SpV/SpV scheme and 4 (17.4%) who received SpV/Mod had a prior confirmed SARS-CoV-2 infection (p = 0.589). Seven out of 23 (30.4%) previously infected patients had SARS-CoV-2 infection between the first and second vaccine dose and two (8.7%) had SARS-CoV-2 infection between the second dose and the humoral response assessment. Table 1 shows detailed characteristics of the study population.
Immunogenicity
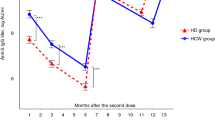
In the overall population, anti-S-RBD IgG was reactive in 103 (96.3%) persons, 80 (95.2%) immunized with the SpV/SpV vaccine, and 23 (100%) with SpV/Mod (p = 0.286). Median (IQR) anti-S-RBD titers were 42.5 (4–1297) BAU/mL after the first dose and 502 (110–1993) BAU/mL after the boost dose (p < 0.001). In participants with a confirmed SARS-CoV-2 infection before complete vaccination receiving the SpV/SpV scheme, the humoral response as measured by anti-S-RBD levels was 6.5-fold higher than that observed in naïve individuals. Similarly, people with a confirmed SARS-CoV-2 infection before the SpV/Mod complete scheme presented 11-fold higher anti-S-RBD levels when compared to participants without prior infection (Fig. 1). Anti-S-RBD levels according to epidemiological and clinical parameters are shown in Table 2. Previous COVID-19 and an SpV/Mod vaccination scheme were independently associated with anti-S-RBD levels in a general linear model (Table 2).
A total of 53 (49.5%) individuals achieved PROT-80. Among participants without prior COVID-19 who received SpV/SpV or SpV/Mod and those with confirmed COVID-19 who received the homologous or the heterologous schemes, PROT-80 rates were 36.9%, 57.9%, 73.7%, and 100% (plinear association < 0.001), respectively. Corresponding values according to epidemiological and clinical parameters are displayed in Table 3. In the multivariate analysis, an independent association with PROT-80 was observed for prior COVID-19, heterologous vaccination, and age in a logistic regression model (Table 3).
Side effects
The homologous and heterologous immunization schemes were well tolerated, and no medical assistance or potentially fatal events were reported. Adverse events, including local and systemic symptoms, were higher for SpV/Mod (47.6%) than for SpV/SpV (23.7%) schemes, p = 0.031. In general, the most frequent systemic adverse events were fatigue (9.9%), myalgia (5.9%), and fever (2.0%). No patients reported headaches, chills, nausea/vomiting, arthralgia, or diarrhea. The heterologous vaccine scheme tended to induce more systemic adverse effects than the homologous one (28.6% vs 15.0%, p = 0.148). Regarding local adverse events, pain at the injection site was reported by 11 patients (10.9%) and tended to be more frequent for the heterologous scheme than for the homologous one (19.0% vs 8.8%, p = 0.178). Prior SARS-CoV-2 infection did not significantly impact on reactogenicity. Thus, 4 (17.4%) and 14 (17.9%) patients with and without previous SARS-CoV-2 infection, respectively, showed systemic symptoms, p = 0.959. Reported local symptoms were 3 (13.0%) for patients with previous SARS-CoV-2 infection and 8 (10.3%) for patients without it, p = 0.706. Figure 2 shows the reactogenicity by adverse effects (local and systemic) according to the vaccination scheme.
Discussion
The present work shows that the implementation of SpV/SpV or SpV/Mod vaccination schemes against SARS-CoV-2 in patients under dialysis was effective, leading to a seroconversion rate of 96.3%. Overall, the heterologous scheme showed an anti-S-RBD level almost threefold higher than the homologous one. Moreover, both vaccination schemes were well tolerated, and no medical assistance was required.
To our knowledge, this is the first study on the heterologous scheme including Sputnik V and Moderna vaccines in naïve and previously SARS-CoV-2 infected patients under dialysis conditions. Additionally, the study allowed results comparison with the homologous SpV/SpV scheme. Findings from our study show the development of a strong humoral response in the dialysis setting in contrast to other studies reporting that patients under dialysis present lower seroconversion rates and anti-S-RBD titers than healthy controls [15]. However, the seroconversion rate of our population under dialysis was similar to that reported by previous studies showing values between 90 and 98% [16,17,18]. Rosa-Diez (2021) detected a 98% seroconversion rate in a study including 102 dialysis patients vaccinated with the Sputnik V scheme [17]. On the other hand, studies performed on patients under dialysis conditions immunized with Moderna vaccines have shown seroconversion rates ranging between 95 and 97.9% [16, 18, 19]. In this context, our results support the use of Sputnik V or its combination with the Moderna vaccine as an alternative for dialysis patients.
Regarding PROT-80, almost 50% of the studied population achieved this threshold. However, the response rates differed significantly when the population was categorized according to age, kidney transplant, vaccine regimen, and prior COVID history. While heterologous scheme and prior SARS-CoV-2 infection were associated with higher PROT-80 proportions, the considerably low 37% PROT-80 rate observed in patients without previous infection and immunized with the SpV/SpV scheme indicate the need for prioritization of a third dose in this particular subpopulation in order to increase and extend antibody levels, especially when further factors that may lower response such as older age or kidney transplant are present.
Concerning the individual factors associated with the humoral response, in the present study, there were no significant anti-S-RBD IgG differences between genders. These findings are consistent with several studies showing that gender does not seem to influence anti-S-RBD IgG titers achieved with both analyzed vaccine schemes [20,21,22]. Likewise, no difference in anti-S-RBD IgG levels according to the age of dialyzed patients was observed in our sample, while an age above 50 years was associated with lower rates of PROT-80. Available data on the relationship between age and response to vaccines are scarce and remain controversial [11, 21, 22]. More studies on this issue are warranted as most reports analyzing the age-associated responses are conducted in healthy populations [11, 21, 22].
Regarding previous infection with SARS-CoV-2, a significant relation was found between higher anti-S-RBD IgG titers and a confirmed past infection for both the SpV/SpV and SpV/MOD schemes, with notable, 11-fold higher levels observed for the heterologous scheme. This finding is in accordance with our previously reported values for these vaccination schemes [11], for the sputnik V homologous scheme [20, 21] and for RNAm vaccines [23].
Additionally, the current study showed that body mass index, time on dialysis, weekly total Kt/V, immunosuppressive therapy, and diabetes as well as other evaluated laboratory parameters, presented no association with Anti-S-RBD levels or PROT-80 rates. Likewise, vaccine efficacy was not associated with ΔP-B, and ΔB-antiSRBD, which is in accordance with findings in the general population immunized with the SpV/SpV and SpV/MOD schemes [11]. While C-reactive protein levels showed an association with Anti-S-RBD and PROT-80 in the univariate analysis, the effect was not maintained after stratifying for other parameters. Additionally, when the Charlson Comorbidity Index was analyzed, no association was found with the vaccine response. There are previous contradictory results regarding this issue. While some authors, including ourselves, could not find an association between these variables and the anti-S-RBD IgG titers [24], other studies suggest that less comorbidity presence leads to a higher anti-S-RBD IgG titer [19, 23]. It is worthy to note that a recent study performed in hemodialysis patients also failed to detect an association between COVID-19 severity and obesity, diabetes, or comorbidity presence [3], factors that do have an impact on disease severity in the overall population. It appears that these differences are also true for vaccine response.
The better immunogenicity of heterologous schemes could be explained by recent evidence showing that mRNA vaccines have a better humoral response when compared to adenovirus-based vaccines [8, 9, 25,26,27]. Furthermore, the enhanced humoral activity induced by the heterologous regimen is correlated with increased frequencies of switched and activated memory B cells recognizing the SARS-CoV-2 RBD [26]. Moreover, the higher anti-S-RBD titers achieved in the SpV/Mod group of this study is in agreement with previous works performed on the general population [11, 28]. However, additional studies are necessary to assess the cellular response since the combination of vaccines appears to enhance the characteristic immune response generated by each vaccination scheme [26, 27].
There is little data on side effects of SpV/SpV or SpV/Mod in dialysis patients and this work offers a contribution to the understanding of SARS-CoV-2 vaccination in this setting. In our sample, adverse events including local and systemic symptoms were higher in dialysis patients who received the SpV/Mod scheme as we have previously described for the general population [11]. In agreement with other studies, fatigue and myalgia were the most frequent systemic complaints [11, 29]. The higher trend in the rate of adverse events in the SpV/Mod group was expected since it is well proven that stronger side effects were associated with mRNA vaccines in the general population [9, 11, 26]. It is important to note that the frequency of adverse events was lower than that observed in the general population and no patients required medical support [11]. However, the median age of the sample analyzed in the present work was higher and the effect of age on the incidence of side effects has already been described in previous studies [3, 13]. Moreover, in a study by Polewska and colleagues (2021), adverse events were less frequently observed in dialyzed patients than in the age and sex-matched control group [29].
This study has some limitations. Firstly, it should be considered that patients were not randomized to receive a particular immunization regimen. Second, patients may have had asymptomatic COVID-19 infection between the two vaccine doses or between the second dose and evaluation of the humoral response. However, it is to be expected that both analyzed groups would have been affected equally; therefore, an impact on the conclusions would unlikely be altered. Third, antibody neutralizing activity was not assessed. Nevertheless, Schmidt et al. showed a significant correlation between IgG levels and neutralizing activity [26]. Lastly, serious adverse effects have been reported with a very low frequency and the small size of the analyzed sample might have influenced this aspect.
In conclusion, both analyzed vaccine schemes were immunogenic, showed a high seroconversion rate and a significant correlation was found between higher anti-S-RBD IgG titers and a confirmed prior infection with SARS-CoV-2 for both schemes. Moreover, the heterologous scheme was also associated with a better humoral response. Finally, both schemes were safe and well-tolerated. These findings should promote patients on dialysis to receive these immunization schemes; however, those who have never been infected with SARS-CoV-2 and received a homologous vaccine scheme may be prioritized for a third dose, especially when more risk factors are present.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, Demertzis Z, Hanna Z, Failla A, Dagher C, Chaudhry Z, Vahia A, Abreu Lanfranco O, Ramesh M, Zervos MJ, Alangaden G, Miller J, Brar I (2020) Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA Netw Open 3(6):e2012270. https://doi.org/10.1001/jamanetworkopen.2020.12270 (PMID: 32543702)
Ozturk S, Turgutalp K, Arici M, Odabas AR, Altiparmak MR, Aydin Z, Cebeci E, Basturk T, Soypacaci Z, Sahin G, Elif Ozler T, Kara E, Dheir H, Eren N, Suleymanlar G, Islam M, Ogutmen MB, Sengul E, Ayar Y, Dolarslan ME, Bakirdogen S, Safak S, Gungor O, Sahin I, Mentese IB, Merhametsiz O, Oguz EG, Genek DG, Alpay N, Aktas N, Duranay M, Alagoz S, Colak H, Adibelli Z, Pembegul I, Hur E, Azak A, Taymez DG, Tatar E, Kazancioglu R, Oruc A, Yuksel E, Onan E, Turkmen K, Hasbal NB, Gurel A, Yelken B, Sahutoglu T, Gok M, Seyahi N, Sevinc M, Ozkurt S, Sipahi S, Bek SG, Bora F, Demirelli B, Oto OA, Altunoren O, Tuglular SZ, Demir ME, Ayli MD, Huddam B, Tanrisev M, Bozaci I, Gursu M, Bakar B, Tokgoz B, Tonbul HZ, Yildiz A, Sezer S, Ates K (2020) Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant 35(12):2083–2095. https://doi.org/10.1093/ndt/gfaa271 (PMID: 33275763)
Selvaskandan H, Hull KL, Adenwalla S, Ahmed S, Cusu MC, Graham-Brown M, Gray L, Hall M, Hamer R, Kanbar A, Kanji H, Lambie M, Lee HS, Mahdi K, Major R, Medcalf JF, Natarajan S, Oseya B, Stringer S, Tabinor M, Burton J (2022) Risk factors associated with COVID-19 severity among patients on maintenance haemodialysis: a retrospective multicentre cross-sectional study in the UK. BMJ Open 12(5):e054869. https://doi.org/10.1136/bmjopen-2021-054869
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, COVE Study Group (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384(5):403–416. https://doi.org/10.1056/NEJMoa2035389 (PMID: 33378609)
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383(27):2603–2615
Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, Botikov AG, Izhaeva FM, Popova O, Ozharovskaya TA, Esmagambetov IB, Favorskaya IA, Zrelkin DI, Voronina DV, Shcherbinin DN, Semikhin AS, Simakova YV, Tokarskaya EA, Egorova DA, Shmarov MM, Nikitenko NA, Gushchin VA, Smolyarchuk EA, Zyryanov SK, Borisevich SV, Naroditsky BS, Gintsburg AL, Gam-COVID-Vac Vaccine Trial Group (2021) Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397(10275):671–681. https://doi.org/10.1016/S0140-6736(21)00234-8
Borobia AM, Carcas AJ, Pérez-Olmeda M, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, Campins M, Portolés A, González-Pérez M, García de la Morales MT, Arana-Arri E, Aldea M, Díez-Fuertes F, Fuentes I, Ascaso A, Lora D, Imaz-Ayo N, Barón-Mira LE, Agustí A, Pérez-Ingidua C, Gómez Cámara A, Arribas JR, Ochando J, Alcamí J, Belda-Iniesta C, Frías J, CombiVacS Study Group (2021) Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 398(10295):121–130. https://doi.org/10.1016/S0140-6736(21)01420-3
Fabricius D, Ludwig C, Scholz J, Rode I, Tsamadou C, Jacobsen EM, Winkelmann M, Grempels A, Lotfi R, Janda A, Körper S, Adler G, Debatin KM, Schrezenmeier H, Jahrsdörfer B (2021) mRNA vaccines enhance neutralizing immunity against SARS-CoV-2 variants in convalescent and ChAdOx1-primed subjects. Vaccines 9(8):918. https://doi.org/10.3390/vaccines9080918
Hillus D, Schwarz T, Tober-Lau P, Vanshylla K, Hastor H, Thibeault C, Jentzsch S, Helbig ET, Lippert LJ, Tscheak P, Schmidt ML, Riege J, Solarek A, von Kalle C, Dang-Heine C, Gruell H, Kopankiewicz P, Suttorp N, Drosten C, Bias H, Seybold J, Klein F, Kurth F, Corman VM, Sander LE, EICOV/COVIM Study Group (2021) Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med 9(11):1255–1265. https://doi.org/10.1016/S2213-2600(21)00357-X
Normark J, Vikström L, Gwon YD, Persson IL, Edin A, Björsell T, Dernstedt A, Christ W, Tevell S, Evander M, Klingström J, Ahlm C, Forsell M (2021) Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med 385(11):1049–1051. https://doi.org/10.1056/NEJMc2110716
Pereson JM, Amaya L, Neukam K, Baré P, Echegoyen N, Badano MN, Lucero A, Martelli A, García GH, Videla C, Martínez AP, Di Lello FA (2022) Heterologous Gam-COVID-Vac (Sputnik V)/mRNA-1273 (Moderna) vaccination induces a stronger humoral response than homologous Sputnik V in a real-world data analysis. Clin Microbiol Inf S1198-743X(22)00265–8. https://doi.org/10.1016/j.cmi.2022.05.009
Galli C, Daghfal D, Averhoff F. Antibody testing for SARS-CoV-2 infection, quantitative determination, response to vaccines and viral variability. https://cdn.pepperapps.io/diagnostics-cms/public/60dcbed551c1ff090981ed95?signature=eyJhbGciOiJkaXIiLCJlbmMiOiJBMTI4Q0JDLUhTMjU2In0..9nFGX43vdCD-Qd2XE-NzdA.e5mgnWdULSy2PGkSwfQ10kEG1UQzLUxIzkdUvU7F1xv06WNo-c47joEl46OgfiQdEoako-TvRl4CwkLYtVIVYRR7v2jcqnkBx9SFQIzw-nqvFqHkx_WlydBAcI4ZA_wEKCPydLqBtvFu7APi9pVFVDt-WE7028r1nMWpvAe5CiYb2tzNgCGlvM09-oxpfdsY.vWBKl-boWE1UxgZgSc1K6Q. Accessed 3 Apr 2022.
Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, Dold C, Fuskova M, Gilbert SC, Hirsch I, Humphries HE, Jepson B, Kelly EJ, Plested E, Shoemaker K, Thomas KM, Vekemans J, Villafana TL, Lambe T, Pollard AJ (2021) Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 27(11):2032–2040. https://doi.org/10.1038/s41591-021-01540-1
Charlson ME, Pompei P, Ales KL, MaKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Yen CC, Lin SY, Chen SC, Chiu YW, Chang JM, Hwang SJ (2021) COVID 19 vaccines in patients with maintenance hemodialysis. J Pers Med 11(8):789. https://doi.org/10.3390/jpm11080789 (PMID: 34442432)
Beilhack G, Monteforte R, Frommlet F, Gaggl M, Strassl R, Vychytil A (2021) Antibody response and safety after mRNA-1273 SARS-CoV-2 vaccination in peritoneal dialysis patients—the Vienna Cohort. Front Immunol 12:780594. https://doi.org/10.3389/fimmu.2021.780594 (PMID: 34925359)
Rosa-Diez G, Papaginovic Leiva MM, Lombi F, Crucelegui MS, Martínez RD, Trimarchi H, Schiavelli R, Grizzo M, Raño M, Heguilén RM, Jones RA, Gonzalez Paganti L, Ferrrari M, Zingoni P, Kjohede V, Geffner JR, Ferrante D, González Bernaldo de Quirós F, Pagotto V. Safety and effectiveness of COVID-19 SPUTNIK V vaccine in dialysis patients. Medrxiv. https://doi.org/10.1101/2021.10.21.21265349
Yau K, Abe KT, Naimark D, Oliver MJ, Perl J, Leis JA, Bolotin S, Tran V, Mullin SI, Shadowitz E, Gonzalez A, Sukovic T, Garnham-Takaoka J, de Launay KQ, Takaoka A, Straus SE, McGeer AJ, Chan CT, Colwill K, Gingras AC, Hladunewich MA (2021) Evaluation of the SARS-CoV-2 antibody response to the BNT162b2 vaccine in patients undergoing hemodialysis. JAMA Netw Open 4:e2123622. https://doi.org/10.1001/jamanetworkopen.2021.23622
Ionita C, Marcelli D, Nita C, Anton C, Berca S, Vacar S, Schiller O, Gheorghiu C, Barth C (2022) Comparison of antibody response to two different mRNA Covid-19 vaccines in patients on hemodialysis. J Nephrol 35(1):143–151. https://doi.org/10.1007/s40620-021-01195-8 (PMID: 34978050)
Claro F, Silva D, Rodriguez M, Rangel HR, de Waard JH (2021) Immunoglobulin G antibody response to the Sputnik V vaccine: previous SARS-CoV-2 seropositive individuals may need just one vaccine dose. Int j Inf Dis 111:261–266. https://doi.org/10.1016/j.ijid.2021.07.070
Rovere P, Laurelli A, Diaz A, Dabusti G, Valdez P (2021) Seroprevalence of anti S1 SARS-CoV-2 antibodies in workers vaccinated with Sputnik V at a public hospital in Buenos Aires. Medicina (B Aires) 81(6):895–901
Wheeler SE, Shurin GV, Yost M, Anderson A, Pinto L, Wells A, Shurin MR (2021) Differential antibody response to mRNA COVID-19 vaccines in healthy subjects. Microbiol Spectrum 9(1):e0034121. https://doi.org/10.1128/Spectrum.00341-21
Cortés-Sarabia K, Gutiérrez-Torres M, Mendoza-Renteria EM, Leyva-Vázquez MA, Vences-Velázquez A, Hernández-Sotelo D, Beltrán-Anaya FO, Del Moral-Hernández O, Illades-Aguiar B (2022) Variation in the humoral immune response induced by the administration of the BNT162b2 Pfizer/BioNTech vaccine: a systematic review. Vaccines (Basel) 10(6):909. https://doi.org/10.3390/vaccines10060909
Duarte R, Roldão M, Figueiredo C, Luz I, Ferrer F, Gonçalves H, Sofia F, Lopes K (2022) Humoral response to BNT162b2 mRNA COVID-19 vaccine in peritoneal and hemodialysis patients: a comparative study. Ther Apher Dial 26(4):790–796. https://doi.org/10.1111/1744-9987.13766
Mahallawi WH, Ibrahim NA, Mumena WA (2021) Effectiveness of COVID-19 vaccines in patients under maintenance hemodialysis. Risk Manag Healthc Policy 14:5081–5088. https://doi.org/10.2147/RMHP.S345686 (PMID: 35002344)
Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, Abu-Omar A, Ziegler L, Guckelmus C, Urschel R, Schneitler S, Becker SL, Gärtner BC, Sester U, Sester M (2021) Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med 27(9):1530–1535. https://doi.org/10.1038/s41591-021-01464-w
Pozzetto B, Legros V, Djebali S, Barateau V, Guibert N, Villard M, Peyrot L, Allatif O, Fassier JB, Massardier-Pilonchéry A, Brengel-Pesce K, Yaugel-Novoa M, Denolly S, Boson B, Bourlet T, Bal A, Valette M, Andrieu T, Lina B, Cosset FL, Paul S, Defrance T, Marvel J, Walzer T, Trouillet-Assant S, Covid-Ser study group (2021) Immunogenicity and efficacy of heterologous ChadOx1/BNT162b2 vaccination. Nature. https://doi.org/10.1038/s41586-021-04120-y.10.1038/s41586-021-04120-y
Macchia A, Ferrante D, Bouzas MB, Angeleri P, Biscayart C, Geffner J, Mammana L, Zapiola I, López EL, Gentile A, Varese A, Mazzitelli I, García FDD, Sharff D, Lucconi V, Sujansky P, Mariani J, de Quirós FGB (2022) Immunogenicity induced by the use of alternative vaccine platforms to deal with vaccine shortages in a low- to middle-income country: results of two randomized clinical trials. Lancet Reg Health Am 9:100196. https://doi.org/10.1016/j.lana.2022.100196 (PMID:35128512)
Polewska K, Tylicki P, Biedunkiewicz B, Rucińska A, Szydłowska A, Kubanek A, Rosenberg I, Rodak S, Ślizień W, Renke M, Dębska-Ślizień A, Tylicki L (2021) Safety and tolerability of the BNT162b2 mRNA COVID-19 vaccine in dialyzed patients. COViNEPH Project Medicina (Kaunas) 57(7):732. https://doi.org/10.3390/medicina57070732 (PMID: 34357013)
Acknowledgements
FAD is a member of the National Research Council (CONICET) Research Career Program. K.N. is the recipient of a Miguel Servet contract by the Instituto de Salud Carlos III (grant number CPII18/00033). We would like to thank Mrs. Silvina Heisecke, from CEMIC‐CONICET, for the copyediting of the manuscript
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
GL: Analysis and interpretation of data, Drafting the article. Final approval of the version to be published. APM: Analysis and interpretation of data, Providing intellectual content of critical importance to the work described. Revising the article. Final approval of the version to be published. WRG: Analysis and interpretation of data, Providing intellectual content of critical importance to the work described. Revising the article. Final approval of the version to be published. LA: Analysis and interpretation of data, Providing intellectual content of critical importance to the work described. Revising the article. Final approval of the version to be published. AA: Analysis and interpretation of data, Providing intellectual content of critical importance to the work described. Revising the article. Final approval of the version to be published. NE: Analysis and interpretation of data, Providing intellectual content of critical importance to the work described. Revising the article. Final approval of the version to be published. CD: Analysis and interpretation of data, Providing intellectual content of critical importance to the work described. Revising the article. Final approval of the version to be published. AL: Analysis and interpretation of data, Providing intellectual content of critical importance to the work described. Revising the article. Final approval of the version to be published. AM: Analysis and interpretation of data, Providing intellectual content of critical importance to the work described. Revising the article. Final approval of the version to be published. CV: Analysis and interpretation of data, Providing intellectual content of critical importance to the work described. Revising the article. Final approval of the version to be published. KN: Analysis and interpretation of data, Providing intellectual content of critical importance to the work described. Drafting the article. Final approval of the version to be published FAL: Conception, design, analysis and interpretation of data. Drafting the article. Final approval of the version to be published.
Corresponding authors
Ethics declarations
Competing interests
On behalf of all authors, the corresponding author states that the authors have no relevant financial or non-financial interests to disclose.
Ethical disclosures
The study was designed and performed according to the Helsinki declaration and all participants gave their written informed consent (Study protocol EX-2021–06438339-UBA DME#SSA_FFYB, Ethics committee of the Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Laham, G., Martínez, A.P., Rojas Gimenez, W. et al. Assessment of the humoral response to the homologous Gam-COVID-Vac (Sputnik V) or heterologous Sputnik V/mRNA-1273 (Moderna) vaccination against SARS-CoV-2 in dialysis patients. J Nephrol 36, 861–872 (2023). https://doi.org/10.1007/s40620-022-01446-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-022-01446-2