Abstract
Purpose
To perform an updated review regarding the influence of methodological conditions on radiomics analyses of 18F-FDG PET imaging.
Methods
We performed a systematic review of the literature using PubMed/Medline and Google scholars, with multiple research keywords for each organ accompanying the terms “radiomics”, “texture”, “heterogeneity”, “FDG”, “PET” and “PET/CT”. The review considers methodological studies for shape, histogram and textural features extracted from FDG PET imaging. The references cited in the retrieved articles were also explored to find additional studies. The search was limited to English language. Preclinical and animal studies were not included in the review. A total of 44 original articles were considered for the review.
Results
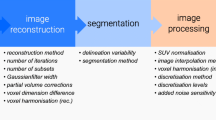
In the same conditions, repeatability is very variable among the radiomics features in FDG PET. The vast majority of features are sensitive to acquisition and reconstruction settings independently of the category or order of features. Similar sensitivity and variability are observed with respect to respiratory motion, pre-processing, segmentation method, and specifically for textural features, discretisation of grey levels and implementation/parameterisation of texture matrices.
Conclusion
Radiomics features are sensitive to almost all factors involved in the PET/CT workflow, from the generation of the images, to the radiomics implementation choices. This strongly supports the need for standardization efforts of these conditions to enable implementation in clinical routine and multicentric studies. However, to date the repercussion of these features fluctuations on the clinical endpoints has been rarely studied. Similar standardization and consensus will also be needed for the statistical analysis and machine learning aspects involved in radiomics analyses of FDG PET images.
Similar content being viewed by others
Abbreviations
- ASM:
-
Angular second moment
- CCC:
-
Concordance correlation coefficient
- CHAUC :
-
Area under the curve of the cumulative histogram
- COV:
-
Coefficient of variation
- FLAB:
-
Fuzzy locally adaptive bayesian algorithm
- GLCM:
-
Grey-level co-occurrence matrix
- GLRLM:
-
Grey-level run-length matrix
- GLZSM:
-
Grey-level zone-size matrix
- HILAE:
-
High-intensity large area emphasis
- HGZE:
-
High grey-level zone emphasis
- ICC:
-
Intra-class correlation coefficients
- IV:
-
Intensity variability
- LGZE:
-
Low grey-level zone emphasis
- LRE:
-
Long-run emphasis
- MTV:
-
Metabolic tumour volume
- NGTDM:
-
Neighbourhood grey-tone difference matrix
- NSCLC:
-
Non-small lung cancer
- OSEM:
-
Ordered subset expectation maximisation
- PSF:
-
Point spread function
- SRE:
-
Short-run emphasis
- SUV:
-
Standardised uptake value
- SUVSD :
-
SUV standard deviation
- SZV:
-
Size-zone variability
- TOF:
-
Time of flight
- VOI:
-
Volume of interest
- ZP:
-
Zone percentage
References
Ganeshan B, Panayiotou E, Burnand K et al (2012) Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 22(4):796–802
O’Connor JP, Rose CJ, Waterton JC et al (2015) Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res 21(2):249–257
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577
Hatt M, Tixier F, Visvikis D et al (2017) Radiomics in PET/CT: more than meets the eye? J Nucl Med 58(3):365–366
El Naqa I, Grigsby P, Apte A et al (2009) Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit 42(6):1162–1171
Tixier F, Le Rest CC, Hatt M et al (2011) Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med 52(3):369–378
Cook GJ, Yip C, Siddique M et al (2013) Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med 54(1):19–26
Aerts HJ, Velazquez ER, Leijenaar RT et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006
Lovinfosse P, Janvary ZL, Coucke P et al (2016) FDG PET/CT texture analysis for predicting the outcome of lung cancer treated by stereotactic body radiation therapy. Eur J Nucl Med Mol Imaging 43(8):1453–1460
Lovinfosse P, Koopmansch B, Lambert F et al (1063) (18)F-FDG PET/CT imaging in rectal cancer: relationship with the RAS mutational status. Br J Radiol 2016(89):20160212
Vallieres M, Kumar A, Sultanem K et al (2016) FDG-PET image-derived features can determine HPV status in head-and-neck cancer. Int J Radiat Oncol Biol Phys 87(2):S467
Yip SS, Kim J, Coroller TP et al (2017) Associations between somatic mutations and metabolic imaging phenotypes in non-small cell lung cancer. J Nucl Med 58(4):569–576
Lovinfosse P, Polus M, Van Daele D et al (2018) FDG PET/CT radiomics for predicting the outcome of locally advanced rectal cancer. Eur J Nucl Med Mol Imaging 45(3):365–375
Wahl RL, Jacene H, Kasamon Y et al (2009) From RECIST to PERCIST: evolving Considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Tixier F, Hatt M, Le Rest CC et al (2012) Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18F-FDG PET. J Nucl Med 53(5):693–700
Leijenaar RT, Carvalho S, Velazquez ER et al (2013) Stability of FDG-PET Radiomics features: an integrated analysis of test-retest and inter-observer variability. Acta Oncol 52(7):1391–1397
van Velden FH, Nissen IA, Jongsma F et al (2014) Test-retest variability of various quantitative measures to characterize tracer uptake and/or tracer uptake heterogeneity in metastasized liver for patients with colorectal carcinoma. Mol Imaging Biol 16(1):13–18
Desseroit MC, Tixier F, Weber WA et al (2017) Reliability of PET/CT shape and heterogeneity features in functional and morphologic components of non-small cell lung cancer tumors: a repeatability analysis in a prospective multicenter cohort. J Nucl Med 58(3):406–411
Galavis PE, Hollensen C, Jallow N et al (2010) Variability of textural features in FDG PET images due to different acquisition modes and reconstruction parameters. Acta Oncol 49(7):1012–1016
Lovat E, Siddique M, Goh V et al (2017) The effect of post-injection (18)F-FDG PET scanning time on texture analysis of peripheral nerve sheath tumours in neurofibromatosis-1. EJNMMI Res 7(1):35
Hustinx R, Smith RJ, Benard F et al (1999) Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med 26(10):1345–1348
Cheng G, Torigian DA, Zhuang H et al (2013) When should we recommend use of dual time-point and delayed time-point imaging techniques in FDG PET? Eur J Nucl Med Mol Imaging 40(5):779–787
Yan J, Chu-Shern JL, Loi HY et al (2015) Impact of image reconstruction settings on texture features in 18F-FDG PET. J Nucl Med 56(11):1667–1673
Orlhac F, Nioche C, Soussan M et al (2017) Understanding changes in tumor texture indices in PET: a comparison between visual assessment and index values in simulated and patient data. J Nucl Med 58(3):387–392
Lasnon C, Majdoub M, Lavigne B et al (2016) (18)F-FDG PET/CT heterogeneity quantification through textural features in the era of harmonisation programs: a focus on lung cancer. Eur J Nucl Med Mol Imaging 43(13):2324–2335
Hatt M, Cheze le Rest C, Descourt P et al (2010) Accurate automatic delineation of heterogeneous functional volumes in positron emission tomography for oncology applications. Int J Radiat Oncol Biol Phys 77(1):301–308
Hatt M, Cheze-le Rest C, van Baardwijk A et al (2011) Impact of tumor size and tracer uptake heterogeneity in (18)F-FDG PET and CT non-small cell lung cancer tumor delineation. J Nucl Med 52(11):1690–1697
van Velden FH, Kramer GM, Frings V et al (2016) Repeatability of radiomic features in non-small-cell lung cancer [(18)F]FDG-PET/CT studies: impact of reconstruction and delineation. Mol Imaging Biol 18(5):788–795
Hatt M, Tixier F, Cheze Le Rest C et al (2013) Robustness of intratumour (1)(8)F-FDG PET uptake heterogeneity quantification for therapy response prediction in oesophageal carcinoma. Eur J Nucl Med Mol Imaging 40(11):1662–1671
Doumou G, Siddique M, Tsoumpas C et al (2015) The precision of textural analysis in (18)F-FDG-PET scans of oesophageal cancer. Eur Radiol 25(9):2805–2812
Grootjans W, Tixier F, van der Vos CS et al (2016) The impact of optimal respiratory gating and image noise on evaluation of intratumor heterogeneity on 18F-FDG PET imaging of lung cancer. J Nucl Med 57(11):1692–1698
Forgacs A, Jonsson HP, Dahlbom M (2016) A study on the basic criteria for selecting heterogeneity parameters of F18-FDG PET images. PLoS One 11(10):e0164113
Shiri I, Rahmim A, Ghaffarian P et al (2017) The impact of image reconstruction settings on 18F-FDG PET radiomic features: multi-scanner phantom and patient studies. Eur Radiol 27(11):4498–4509
Yip SS, Aerts HJ (2016) Applications and limitations of radiomics. Phys Med Biol 61(13):R150–R166
Johnson WE, Li C, Rabinovic A (2007) Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8(1):118–127
Muller C, Schillert A, Rothemeier C et al (2016) Removing batch effects from longitudinal gene expression—quantile normalization plus ComBat as best approach for microarray transcriptome data. PLoS One 11(6):e0156594
Goh WWB, Wang W, Wong L (2017) Why batch effects matter in omics data, and how to avoid them. Trends Biotechnol 35(6):498–507
Orlhac F, Boughdad S, Philippe C et al. (2018) A post-reconstruction harmonization method for multicenter radiomic studies in PET. J Nucl Med
Fortin JP, Parker D, Tunc B et al (2017) Harmonization of multi-site diffusion tensor imaging data. Neuroimage 161:149–170
Cheng NM, Fang YH, Yen TC (2013) The promise and limits of PET texture analysis. Ann Nucl Med 27(9):867–869
Hahn DA, Daum V, Hornegger J (2010) Automatic parameter selection for multimodal image registration. IEEE Trans Med Imaging 29(5):1140–1155
Cheng NM, Fang YH, Chang JT et al (2013) Textural features of pretreatment 18F-FDG PET/CT images: prognostic significance in patients with advanced T-stage oropharyngeal squamous cell carcinoma. J Nucl Med 54(10):1703–1709
Orlhac F, Soussan M, Maisonobe JA et al (2014) Tumor texture analysis in 18F-FDG PET: relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med 55(3):414–422
Leijenaar RT, Nalbantov G, Carvalho S et al (2015) The effect of SUV discretization in quantitative FDG-PET radiomics: the need for standardized methodology in tumor texture analysis. Sci Rep 5:11075
Orlhac F, Soussan M, Chouahnia K et al (2015) 18F-FDG PET-derived textural indices reflect tissue-specific uptake pattern in non-small cell lung cancer. PLoS One 10(12):e0145063
Lu L, Lv W, Jiang J et al (2016) Robustness of radiomic features in [(11)C]choline and [(18)F]FDG PET/CT imaging of nasopharyngeal carcinoma: impact of segmentation and discretization. Mol Imaging Biol 18(6):935–945
Brooks FJ, Grigsby PW (2014) The effect of small tumor volumes on studies of intratumoral heterogeneity of tracer uptake. J Nucl Med 55(1):37–42
Hatt M, Majdoub M, Vallieres M et al (2015) 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med 56(1):38–44
Vallieres M, Freeman CR, Skamene SR et al (2015) A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol 60(14):5471–5496
Hatt M, Lee JA, Schmidtlein CR et al (2017) Classification and evaluation strategies of auto-segmentation approaches for PET: report of AAPM task group No. 211. Med Phys 44(6):e1–e42
Schinagl DA, Vogel WV, Hoffmann AL et al (2007) Comparison of five segmentation tools for 18F-fluoro-deoxy-glucose-positron emission tomography-based target volume definition in head and neck cancer. Int J Radiat Oncol Biol Phys 69(4):1282–1289
Vees H, Senthamizhchelvan S, Miralbell R et al (2009) Assessment of various strategies for 18F-FET PET-guided delineation of target volumes in high-grade glioma patients. Eur J Nucl Med Mol Imaging 36(2):182–193
Zaidi H, El Naqa I (2010) PET-guided delineation of radiation therapy treatment volumes: a survey of image segmentation techniques. Eur J Nucl Med Mol Imaging 37(11):2165–2187
Hatt M, Laurent B, Ouahabi A et al (2018) The first MICCAI challenge on PET tumor segmentation. Med Image Anal 44:177–195
Hatt M, Laurent B, Fayad H et al (2018) Tumour functional sphericity from PET images: prognostic value in NSCLC and impact of delineation method. Eur J Nucl Med Mol Imaging 45(4):630–641
Nestle U, Kremp S, Schaefer-Schuler A et al (2005) Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J Nucl Med 46(8):1342–1348
Frings V, van Velden FH, Velasquez LM et al (2014) Repeatability of metabolically active tumor volume measurements with FDG PET/CT in advanced gastrointestinal malignancies: a multicenter study. Radiology 273(2):539–548
Tian J, Xue J, Dai Y et al (2008) A novel software platform for medical image processing and analyzing. IEEE Trans Inf Technol Biomed 12(6):800–812
Adams R, Bischof L (1994) Seeded region growing. IEEE Trans Pattern Anal Mach Intell 16(6):641–647
Hatt M, Visvikis D, Pradier O et al (2011) Baseline (1)(8)F-FDG PET image-derived parameters for therapy response prediction in oesophageal cancer. Eur J Nucl Med Mol Imaging 38(9):1595–1606
Hatt M, Cheze le Rest C, Turzo A et al (2009) A fuzzy locally adaptive Bayesian segmentation approach for volume determination in PET. IEEE Trans Med Imaging 28(6):881–893
Parmar C, Velazquez ER, Leijenaar R (2014) Robust radiomics feature quantification using semiautomatic volumetric segmentation. PLoS One 9(7):e102107
Yip SS, Coroller TP, Sanford NN et al (2016) Use of registration-based contour propagation in texture analysis for esophageal cancer pathologic response prediction. Phys Med Biol 61(2):906–922
Hsu CY, Doubrovin M, Hua CH et al (2018) Radiomics features differentiate between normal and tumoral high-Fdg uptake. Sci Rep 8(1):3913
Yip S, McCall K, Aristophanous M et al (2014) Comparison of texture features derived from static and respiratory-gated PET images in non-small cell lung cancer. PLoS One 9(12):e115510
Oliver JA, Budzevich M, Zhang GG et al (2015) Variability of image features computed from conventional and respiratory-gated PET/CT images of lung cancer. Transl Oncol 8(6):524–534
Vaidya M, Creach KM, Frye J et al (2012) Combined PET/CT image characteristics for radiotherapy tumor response in lung cancer. Radiother Oncol 102(2):239–245
Lemarignier C, Martineau A, Teixeira L et al (2017) Correlation between tumour characteristics, SUV measurements, metabolic tumour volume, TLG and textural features assessed with (18)F-FDG PET in a large cohort of oestrogen receptor-positive breast cancer patients. Eur J Nucl Med Mol Imaging 44(7):1145–1154
O’Connor JP, Aboagye EO, Adams JE et al (2017) Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 14(3):169–186
Zwanenburg A, Leger S, Vallières M et al. (2016) Image biomarker standardisation initiative. arXiv: 161207003
Parmar C, Grossmann P, Bussink J et al (2015) Machine learning methods for quantitative radiomic biomarkers. Sci Rep 5:13087
Ypsilantis PP, Siddique M, Sohn HM et al (2015) Predicting response to neoadjuvant chemotherapy with PET imaging using convolutional neural networks. PLoS One 10(9):e0137036
Chicco D (2017) Ten quick tips for machine learning in computational biology. BioData Min 10:35
Folkert MR, Setton J, Apte AP et al (2017) Predictive modeling of outcomes following definitive chemoradiotherapy for oropharyngeal cancer based on FDG-PET image characteristics. Phys Med Biol 62(13):5327–5343
Leger S, Zwanenburg A, Pilz K et al (2017) A comparative study of machine learning methods for time-to-event survival data for radiomics risk modelling. Sci Rep 7(1):13206
Carvalho S, Leijenaar RTH, Troost EGC et al (2018) 18F-fluorodeoxyglucose positron-emission tomography (FDG-PET)-radiomics of metastatic lymph nodes and primary tumor in non-small cell lung cancer (NSCLC)—a prospective externally validated study. PLoS One 13(3):e0192859
Author information
Authors and Affiliations
Contributions
PL: Content planning, literature search and review, manuscript writing. DV: Content planning, manuscript editing. RH: Content planning, manuscript editing. MH: Content planning, manuscript writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lovinfosse, P., Visvikis, D., Hustinx, R. et al. FDG PET radiomics: a review of the methodological aspects. Clin Transl Imaging 6, 379–391 (2018). https://doi.org/10.1007/s40336-018-0292-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-018-0292-9