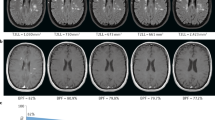
Abstract
Multiple sclerosis (MS) is a chronic disease with an inflammatory and neurodegenerative pathology. Axonal loss and neurodegeneration occurs early in the disease course and may lead to irreversible neurological impairment. Changes in brain volume, observed from the earliest stage of MS and proceeding throughout the disease course, may be an accurate measure of neurodegeneration and tissue damage. There are a number of magnetic resonance imaging-based methods for determining global or regional brain volume, including cross-sectional (e.g. brain parenchymal fraction) and longitudinal techniques (e.g. SIENA [Structural Image Evaluation using Normalization of Atrophy]). Although these methods are sensitive and reproducible, caution must be exercised when interpreting brain volume data, as numerous factors (e.g. pseudoatrophy) may have a confounding effect on measurements, especially in a disease with complex pathological substrates such as MS. Brain volume loss has been correlated with disability progression and cognitive impairment in MS, with the loss of grey matter volume more closely correlated with clinical measures than loss of white matter volume. Preventing brain volume loss may therefore have important clinical implications affecting treatment decisions, with several clinical trials now demonstrating an effect of disease-modifying treatments (DMTs) on reducing brain volume loss. In clinical practice, it may therefore be important to consider the potential impact of a therapy on reducing the rate of brain volume loss. This article reviews the measurement of brain volume in clinical trials and practice, the effect of DMTs on brain volume change across trials and the clinical relevance of brain volume loss in MS.
Similar content being viewed by others
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–31.
Dutta R, Trapp BD. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol. 2011;93:1–12.
Young CA. Factors predisposing to the development of multiple sclerosis. QJM. 2011;104:383–6.
Siffrin V, Vogt J, Radbruch H, et al. Multiple sclerosis—candidate mechanisms underlying CNS atrophy. Trends Neurosci. 2010;33:202–10.
Bar-Or A. The immunology of multiple sclerosis. Semin Neurol. 2008;28:29–45.
Barten LJ, Allington DR, Procacci KA, et al. New approaches in the management of multiple sclerosis. Drug Des Devel Ther. 2010;4:343–66.
Filippi M, Rocca MA, Barkhof F, et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012;11:349–60.
Filippi M, Rocca M. Preventing brain atrophy should be the gold standard of effective theraphy in MS (after the first year of treatment): no. Mult Scler. 2013;19:1005–6.
Rudick RA, Fisher E. Preventing brain atrophy should be the gold standard of effective therapy in MS (after the first year of treatment): yes. Mult Scler. 2013;19:1003–4.
Arnold D, De Stefano N. Preventing brain atrophy should be the gold standard of effective therapy in multiple sclerosis (after the first year of treatment): commentary. Mult Scler. 2013;19:1007–8.
Barkhof F, Calabresi PA, Miller DH, et al. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–66.
Zivadinov R, Bakshi R. Central nervous system atrophy and clinical status in multiple sclerosis. J Neuroimaging. 2004;14(3 Suppl.):27S–35S.
Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5:158–70.
Giorgio A, Battaglini M, Smith SM, et al. Brain atrophy assessment in multiple sclerosis: importance and limitations. Neuroimaging Clin N Am. 2008;18:675–86.
Zipp F. A new window in multiple sclerosis pathology: non-conventional quantitative magnetic resonance imaging outcomes. J Neurol Sci. 2009;287(Suppl 1):S24–9.
Enzinger C, Fazekas F, Matthews PM, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64:1704–11.
Zivadinov R, Weinstock-Guttman B, Hashmi K, et al. Smoking is associated with increased lesion volumes and brain atrophy in multiple sclerosis. Neurology. 2009;73:504–10.
Durand-Dubief F, Belaroussi B, Armspach JP, et al. Reliability of longitudinal brain volume loss measurements between 2 sites in patients with multiple sclerosis: comparison of 7 quantification techniques. Am J Neuroradiol. 2012;33:1918–24.
Rudick RA, Fisher E, Lee JC, et al. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology. 1999;53:1698–704.
Filippi M, Rovaris M, Inglese M, et al. Interferon beta-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:1489–96.
Kappos L, Freedman MS, Polman CH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 2009;8:987–97.
Molyneux PD, Kappos L, Polman C, et al. The effect of interferon beta-1b treatment on MRI measures of cerebral atrophy in secondary progressive multiple sclerosis. European Study Group on Interferon beta-1b in secondary progressive multiple sclerosis. Brain. 2000;123(Pt 11):2256–63.
Sormani MP, Rovaris M, Valsasina P, et al. Measurement error of two different techniques for brain atrophy assessment in multiple sclerosis. Neurology. 2004;62:1432–4.
Comi G, Martinelli V, Rodegher M, et al. Effects of early treatment with glatiramer acetate in patients with clinically isolated syndrome. Mult Scler. 2013;19:1074–83.
Vidal-Jordana A, Sastre-Garriga J, Pérez-Miralles F, et al. Early brain pseudoatrophy while on natalizumab therapy is due to white matter volume changes. Mult Scler. 2013;19:1175–81.
Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68:1390–401.
Barkhof F, Hulst HE, Drulovic J, et al. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology. 2010;74:1033–40.
Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401.
O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–303.
Comi G, Jeffery D, Kappos L, et al. Placebo-controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med. 2012;366:1000–9.
Filippi M, Rocca MA, Pagani E, et al. Placebo-controlled trial of oral laquinimod in multiple sclerosis: MRI evidence of an effect on brain tissue damage. J Neurol Neurosurg Psychiatry. Epub 12 Sep 2013.
Vollmer TL, Soelberg Sorensen P, Arnold DL, et al. A placebo-controlled and active comparator phase III trial (BRAVO) for relapsing–remitting multiple sclerosis. ECTRIMS 2011. Abstract P148.
Arnold DL, Gold R, Kappos L, et al. Effects of BG-12 on magnetic resonance imaging outcomes in the DEFINE study. CMSC 2012. Poster DX21.
Miller D, Fox RJ, Phillips JT, et al. Effects of BG-12 on magnetic resonance imaging outcomes in CONFIRM (Comparator and an Oral Fumarate in Relapsing-Remitting Multiple Sclerosis), a randomized, placebo-controlled, phase 3 study. ENS 2012. O259.
O’Connor P, Filippi M, Arnason B, et al. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 2009;8:889–97.
Hardmeier M, Wagenpfeil S, Freitag P, et al. Rate of brain atrophy in relapsing MS decreases during treatment with IFN beta-1a. Neurology. 2005;64:236–40.
Borges IT, Shea CD, Ohayon J, et al. The effect of daclizumab on brain atrophy in relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2013;2:133–40.
Bendfeldt K, Egger H, Nichols TE, et al. Effect of immunomodulatory medication on regional gray matter loss in relapsing-remitting multiple sclerosis—a longitudinal MRI study. Brain Res. 2010;1325:174–82.
Khan O, Bao F, Shah M, et al. Effect of disease-modifying therapies on brain volume in relapsing-remitting multiple sclerosis: results of a five-year brain MRI study. J Neurol Sci. 2012;312:7–12.
Portaccio E, Stromillo ML, Goretti B, et al. Natalizumab may reduce cognitive changes and brain atrophy rate in relapsing-remitting multiple sclerosis: a prospective, non-randomized pilot study. Eur J Neurol. 2013;20:986–90.
CAMMS223 Trial Investigators, Coles AJ, Compston DA, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–801.
Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–39.
Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–28.
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15.
Takao H, Hayashi N, Ohtomo K. A longitudinal study of brain volume changes in normal aging. Eur J Radiol. 2012;81:2801–4.
Sidaros A, Skimminge A, Liptrot MG, et al. Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. Neuroimage. 2009;44:1–8.
Scahill RI, Frost C, Jenkins R, et al. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–94.
Hedman AM, van Haren NE, Schnack HG, et al. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33:1987–2002.
Giorgio A, Stromillo ML, Bartolozzi ML, et al. Ten-year brain atrophy and disability changes in multiple sclerosis. AAN 2012. Poster P065.
De Stefano N, Giorgio A, Battaglini M, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74:1868–76.
Fisher E, Lee JC, Nakamura K, et al. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64:255–65.
Simon JH. Brain atrophy in multiple sclerosis: what we know and would like to know. Mult Scler. 2006;12:679–87.
Minneboo A, Jasperse B, Barkhof F, et al. Predicting short-term disability progression in early multiple sclerosis: added value of MRI parameters. J Neurol Neurosurg Psychiatry. 2008;79:917–23.
Amato MP, Portaccio E, Goretti B, et al. Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol. 2007;64:1157–61.
Fisniku LK, Altmann DR, Cercignani M, et al. Magnetization transfer ratio abnormalities reflect clinically relevant grey matter damage in multiple sclerosis. Mult Scler. 2009;15:668–77.
Filippi M, Rocca MA. MR imaging of multiple sclerosis. Radiology. 2011;259:659–81.
Zivadinov R, Havrdová E, Bergsland N, et al. Thalamic atrophy is associated with development of clinically definite multiple sclerosis. Radiology. 2013;268:831–41.
Popescu V, Agosta F, Hulst HE, et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2013;84:1082–91.
Zivadinov R, Bergsland N, Dolezal O, et al. Evolution of cortical and thalamus atrophy and disability progression in early relapsing-remitting MS during 5 years. Am J Neuroradiol. 2013;34:1931–9.
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247–54.
Sanfilipo MP, Benedict RH, Sharma J, et al. The relationship between whole brain volume and disability in multiple sclerosis: a comparison of normalized gray vs. white matter with misclassification correction. Neuroimage. 2005;26:1068–77.
Fisher E, Rudick RA, Cutter G, et al. Relationship between brain atrophy and disability: an 8-year follow-up study of multiple sclerosis patients. Mult Scler. 2000;6:373–7.
Shiee N, Bazin PL, Zackowski KM, et al. Revisiting brain atrophy and its relationship to disability in multiple sclerosis. PLoS One. 2012;7:e37049.
Lansley J, Mataix-Cols D, Grau M, et al. Localized grey matter atrophy in multiple sclerosis: a meta-analysis of voxel-based morphometry studies and associations with functional disability. Neurosci Biobehav Rev. 2013;37:819–30.
Zivadinov R, Tekwe C, Bergsland N, et al. Bimonthly evolution of cortical atrophy in early relapsing–remitting multiple sclerosis over 2 years: a longitudinal study. Mult Scler Int. 2013;2013:231345.
Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology. 2002;59:1412–20.
Brex PA, Jenkins R, Fox NC, et al. Detection of ventricular enlargement in patients at the earliest clinical stage of MS. Neurology. 2000;54:1689–91.
Dalton CM, Brex PA, Jenkins R, et al. Progressive ventricular enlargement in patients with clinically isolated syndromes is associated with the early development of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;73:141–7.
Pérez-Miralles F, Sastre-Garriga J, Tintoré M, et al. Clinical impact of early brain atrophy in clinically isolated syndromes. Mult Scler. 2013;19:1878–86.
Ingle GT, Stevenson VL, Miller DH, et al. Two-year follow-up study of primary and transitional progressive multiple sclerosis. Mult Scler. 2002;8:108–14.
Rudick RA, Fisher E, Lee JC, et al. Brain atrophy in relapsing multiple sclerosis: relationship to relapses, EDSS, and treatment with interferon beta-1a. Mult Scler. 2000;6:365–72.
Lavorgna L, Bonavita S, Ippolito D, et al. Clinical and magnetic resonance imaging predictors of disease progression in multiple sclerosis: a nine-year follow-up study. Mult Scler. Epub 9 Jul 2013.
Kearney H, Rocca M, Valsasina P, et al. Magnetic resonance imaging correlates of physical disability in relapse onset multiple sclerosis of long disease duration. Mult Scler. 2014;20:72–80.
Rocca MA, Valsasina P, Damjanovic D, et al. Voxel-wise mapping of cervical cord damage in multiple sclerosis patients with different clinical phenotypes. J Neurol Neurosurg Psychiatry. 2013;84:35–41.
Lukas C, Sombekke MH, Bellenberg B, et al. Relevance of spinal cord abnormalities to clinical disability in multiple sclerosis: MR imaging findings in a large cohort of patients. Radiology. 2013;269:542–52.
Valsasina P, Rocca MA, Horsfield MA, et al. Regional cervical cord atrophy and disability in multiple sclerosis: a voxel-based analysis. Radiology. 2013;266:853–61.
Lukas C, Minneboo A, de Groot V, et al. Early central atrophy rate predicts 5 year clinical outcome in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010;81:1351–6.
Horakova D, Dwyer MG, Havrdova E, et al. Gray matter atrophy and disability progression in patients with early relapsing-remitting multiple sclerosis: a 5-year longitudinal study. J Neurol Sci. 2009;282:112–9.
Rocca MA, Mesaros S, Pagani E, et al. Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology. 2010;257:463–9.
Filippi M, Preziosa P, Copetti M, et al. Gray matter damage predicts the accumulation of disability 13 years later in MS. Neurology. 2013;81:1759–67.
Covey TJ, Zivadinov R, Shucard JL, et al. Information processing speed, neural efficiency, and working memory performance in multiple sclerosis: differential relationships with structural magnetic resonance imaging. J Clin Exp Neuropsychol. 2011;33:1129–45.
Nocentini U, Bozzali M, Spanó B, et al. Exploration of the relationships between regional grey matter atrophy and cognition in multiple sclerosis. Brain Imaging Behav. Epub 15 May 2012.
Benedict RH, Hulst HE, Bergsland N, et al. Clinical significance of atrophy and white matter mean diffusivity within the thalamus of multiple sclerosis patients. Mult Scler. 2013;19:1478–84.
Deloire MS, Ruet A, Hamel D, et al. MRI predictors of cognitive outcome in early multiple sclerosis. Neurology. 2011;76:1161–7.
Calabrese M, Rinaldi F, Grossi P, et al. Cortical pathology and cognitive impairment in multiple sclerosis. Expert Rev Neurother. 2011;11:425–32.
Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–41.
Amato MP, Bartolozzi ML, Zipoli V, et al. Neocortical volume decrease in relapsing-remitting MS patients with mild cognitive impairment. Neurology. 2004;63:89–93.
Hulst HE, Steenwijk MD, Versteeg A, et al. Cognitive impairment in MS: impact of white matter integrity, gray matter volume, and lesions. Neurology. 2013;80:1025–32.
Rossi F, Giorgio A, Battaglini M, et al. Relevance of brain lesion location to cognition in relapsing multiple sclerosis. PLoS One. 2012;7:e44826.
Kapoor R, Furby J, Hayton T, et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:681–8.
Zivadinov R, Reder AT, Filippi M, et al. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology. 2008;71:136–44.
Gauthier SA, Berger AM, Liptak Z, et al. Rate of brain atrophy in benign vs early multiple sclerosis. Arch Neurol. 2009;66:234–7.
Romero JR, Vasan RS, Beiser AS, et al. Association of matrix metalloproteinases with MRI indices of brain ischemia and aging. Neurobiol Aging. 2010;31:2128–35.
Bernal F, Elias B, Hartung HP, et al. Regulation of matrix metalloproteinases and their inhibitors by interferon-beta: a longitudinal study in multiple sclerosis patients. Mult Scler. 2009;15:721–7.
Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–8.
Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve in multiple sclerosis: what you’ve got and how you use it. Neurology. 2013;80:2186–93.
Amato MP, Razzolini L, Goretti B, et al. Cognitive reserve and cortical atrophy in multiple sclerosis: a longitudinal study. Neurology. 2013;80:1728–33.
Feinstein A, Lapshin H, O’Connor P, et al. Sub-threshold cognitive impairment in multiple sclerosis: the association with cognitive reserve. J Neurol. 2013;260:2256–61.
Booth AJ, Rodgers JD, Schwartz CE, et al. Active cognitive reserve influences the regional atrophy to cognition link in multiple sclerosis. J Int Neuropsychol Soc. 2013;19:1128–33.
Zivadinov R. Steroids and brain atrophy in multiple sclerosis. J Neurol Sci. 2005;233:73–81.
Barkhof F, Simon JH, Fazekas F, et al. MRI monitoring of immunomodulation in relapse-onset multiple sclerosis trials. Nat Rev Neurol. 2011;8:13–21.
Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol. Epub 5 Sep 2013.
Acknowledgments
Editorial assistance was provided by Daniel Johnson and Katrin Schulz of Health Interactions, which included developing the outline and first draft of the manuscript under the guidance of the corresponding author and collecting and incorporating comments from all authors. This assistance was exclusively funded by Novartis Pharma AG.
Conflict of interest
Dr. De Stefano has served on scientific advisory boards and steering committees of clinical trials for Merck Serono S.A., Teva and Novartis Pharma AG, and has received support for congress participation or speaker honoraria from Biogen Idec, Novartis Pharma AG, Sanofi-Aventis and Teva. He has also received speaker honoraria from Biogen Idec, Merck Serono S.A., Bayer-Schering AG, Teva, Sanofi-Aventis and Novartis Pharma AG.
Dr. Airas has been involved in contract research through agreements between the institution and the sponsor with Novartis, Roche, GE Healthcare, Biogen Idec and Merck Serono S.A. She has received support for congress participation or speaker honoraria from Biogen Idec, Novartis Pharma AG, Sanofi-Aventis, Teva, Merck Serono S.A. and Bayer-Schering AG.
Dr. Grigoriadis has received honoraria from Novartis Pharma AG as a member of the NeuroNet advisory board and as a speaker in scientific meetings organized by Novartis Pharma AG.
Dr. Mattle, or his institution, has received speaker and consulting fees and educational and research grants from AstraZeneca, Bayer, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Merck Sharp & Dohme-Chibret, Novartis Pharma AG, Pfizer, Sanofi-Aventis, Schering AG, Merck, Servier, St. Jude Medical, Vifor Pharma, the Swiss Society of Hypertension, the Swiss Heart Foundation and the Swiss National Science Foundation.
Dr. O’Riordan has been involved in clinical trials as principal investigator with Novartis Pharma AG, Biogen Idec, Schering AG and Teva. He has also been guest lecturer and consultant advisor in medical advisory committees for Novartis Pharma AG, Biogen Idec, Schering AG and Teva.
Dr. Oreja-Guevara has received honoraria as consultant in scientific advisory boards by Bayer-Schering AG, Merck Serono S.A., Biogen Idec, Teva and Novartis Pharma AG and has also participated in clinical trials and other research projects promoted by Biogen Idec, GlaxoSmithKline, Teva and Novartis Pharma AG.
Dr. Sellebjerg has served on scientific advisory boards for Biogen Idec, Genzyme, Merck Serono S.A., Novartis Pharma AG, Sanofi-Aventis and Teva. He has also been on the steering committee of a clinical trial sponsored by Merck Serono S.A., and served as consultant for Biogen Idec and Novo Nordisk. He has received support for congress participation from Biogen Idec, Novartis Pharma AG, Sanofi-Aventis and Teva, and he has also received speaker honoraria from Bayer-Schering AG, Biogen Idec, Genzyme, Merck Serono S.A., Novartis Pharma AG, Sanofi-Aventis and Schering-Plough.
Dr. Stankoff has received lecture fees and served as consultant and on advisory boards for Biogen Idec, Teva, Novartis Pharma AG, Genzyme, Merck Serono S.A., Bayer-Schering AG and Sanofi-Aventis.
Dr. Walczak has received a consulting fee from Novartis Pharma AG for participation in a NeuroNet advisory board.
Dr. Wiendl has received personal compensation for activities with Bayer Healthcare, Biogen Idec/Elan, Sanofi-Aventis, EMD Serono, Teva Neurosciences and Novo Nordisk as a speaker or consultant or as research support. He has received grants and contracts from Bayer Vital, Biogen Idec, Merck Serono S.A., Novartis Pharma AG, Novo Nordisk and Sanofi-Aventis.
Dr. Kieseier has received honoraria for lecturing, travel expenses for attending meetings and financial support for research from Bayer HealthCare, Biogen Idec, Genzyme/Sanofi-Aventis, Grifols, Merck Serono S.A., Mitsubishi Europe, Novartis Pharma AG, Roche, Talecris Plasma Resources and Teva.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Stefano, N., Airas, L., Grigoriadis, N. et al. Clinical Relevance of Brain Volume Measures in Multiple Sclerosis. CNS Drugs 28, 147–156 (2014). https://doi.org/10.1007/s40263-014-0140-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0140-z