Abstract
Purpose of Review
Current clinical efforts to predict and prevent preterm birth are primarily focused on the mother and have made minimal progress in improving outcomes. However, recent data indicate that paternal factors can also influence timing of birth. Herein, we will review recent human and murine data examining the contribution of the father to pregnancy outcomes with an emphasis on environmental exposures that can negatively impact fertility and the timing of birth.
Recent Findings
Human epidemiology studies now clearly indicate that a variety of paternal factors (age, race, weight, smoking status) can influence sperm quality, birth timing and, in some studies, offspring health. Utilizing a mouse model, our data have demonstrated that developmental exposure of the male to the environmental toxicant TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) is associated with a transgenerational reduction in sperm number and quality and an increased risk of preterm birth in an unexposed partner.
Summary
Toxicant exposure history can clearly influence sperm quality in men and mice. Murine data further indicate that exposures which negatively affect sperm quality also impair placental function, potentially leading to preterm birth and other adverse outcomes. Of particular concern, these changes have been linked to epigenetic alterations within the male germ cell which can then be transmitted across multiple generations. Since it is not possible to prevent an ancestral toxicant exposure in a human population, identifying lifestyle modifications that can be implemented during the preconception period to improve sperm quality should be explored for the therapeutic potential to reduce the incidence of PTB and its sequelae.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): The National Academies Collection: Reports funded by National Institutes of Health; 2007.
MarchOfDimes. Premature birth report CARD. In: 2018; 2018. https://www.marchofdimes.org/mission/prematurity-reportcard-tv.aspx. 23 May 19
• Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23(6):646–59. https://doi.org/10.1093/humupd/dmx022 This is a comprehensive meta-analysis identified declining sperm counts among men from North America, Europe and Australia during 1973–2011. Significantly, they identified a 50–60% decline among men unselected by fertility.
Bruner-Tran KL, Gnecco J, Ding T, Glore DR, Pensabene V, Osteen KG. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: translating lessons from murine models. Reprod Toxicol. 2017;68:59–71. https://doi.org/10.1016/j.reprotox.2016.07.007.
Ding T, McConaha M, Boyd KL, Osteen KG, Bruner-Tran KL. Developmental dioxin exposure of either parent is associated with an increased risk of preterm birth in adult mice. Reprod Toxicol. 2011;31(3):351–8. https://doi.org/10.1016/j.reprotox.2010.11.003.
Melnick R, Lucier G, Wolfe M, Hall R, Stancel G, Prins G, et al. Summary of the National Toxicology Program's report of the endocrine disruptors low-dose peer review. Environ Health Perspect. 2002;110(4):427–31. https://doi.org/10.1289/ehp.02110427.
Tiffon C. The impact of nutrition and environmental epigenetics on human health and disease. Int J Mol Sci. 2018;19(11). https://doi.org/10.3390/ijms19113425.
Ding T, Lambert LA, Aronoff DM, Osteen KG, Bruner-Tran KL. Sex-dependent influence of developmental toxicant exposure on group B streptococcus-mediated preterm birth in a murine model. Reprod Sci. 2018;25(5):662–73. https://doi.org/10.1177/1933719117741378.
Ding T, Mokshagundam S, Rinaudo PF, Osteen KG, Bruner-Tran KL. Paternal developmental toxicant exposure is associated with epigenetic modulation of sperm and placental Pgr and Igf2 in a mouse model. Biol Reprod. 2018;99(4):864–76. https://doi.org/10.1093/biolre/ioy111.
Bruner-Tran KL, Resuehr D, Ding T, Lucas JA, Osteen KG. The role of endocrine disruptors in the epigenetics of reproductive disease and dysfunction: potential relevance to humans. Curr Obstet Gynecol Rep. 2012;1(3):116–23. https://doi.org/10.1007/s13669-012-0014-7.
Skinner MK. Endocrine disruptors in 2015: epigenetic transgenerational inheritance. Nat Rev Endocrinol. 2016;12(2):68–70. https://doi.org/10.1038/nrendo.2015.206.
Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Commun. 2013;4:2889. https://doi.org/10.1038/ncomms3889.
Siklenka K, Erkek S, Godmann M, Lambrot R, McGraw S, Lafleur C, et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science. 2015;350(6261):aab2006. https://doi.org/10.1126/science.aab2006.
Estill MS, Krawetz SA. The epigenetic consequences of paternal exposure to environmental contaminants and reproductive toxicants. Curr Environ Health Rep. 2016;3(3):202–13. https://doi.org/10.1007/s40572-016-0101-4.
Ibrahim Y, Hotaling J. Sperm epigenetics and its impact on male fertility, pregnancy loss, and somatic health of future Offsprings. Semin Reprod Med. 2018;36(3–04):233–9. https://doi.org/10.1055/s-0038-1677047.
Barton SC, Adams CA, Norris ML, Surani MA. Development of gynogenetic and parthenogenetic inner cell mass and trophectoderm tissues in reconstituted blastocysts in the mouse. J Embryol Exp Morphol. 1985;90:267–85.
Maltepe E, Fisher SJ. Placenta: the forgotten organ. Annu Rev Cell Dev Biol. 2015;31:523–52. https://doi.org/10.1146/annurev-cellbio-100814-125620.
Wang X, Miller DC, Harman R, Antczak DF, Clark AG. Paternally expressed genes predominate in the placenta. Proc Natl Acad Sci U S A. 2013;110(26):10705–10. https://doi.org/10.1073/pnas.1308998110.
Houben ML, Nikkels PG, van Bleek GM, Visser GH, Rovers MM, Kessel H, et al. The association between intrauterine inflammation and spontaneous vaginal delivery at term: a cross-sectional study. PLoS One. 2009;4(8):e6572. https://doi.org/10.1371/journal.pone.0006572.
Menon R, Richardson LS, Lappas M. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta. 2019;79:40–5. https://doi.org/10.1016/j.placenta.2018.11.003.
Harris WS. Recent trials challenge the benefits of Omega-3. Keio J Med. 2015;64(4):65. https://doi.org/10.2302/kjm.64-003-ABST.
Jordan RG. The challenge of preterm birth. J Midwifery Womens Health. 2008;53(1):96.
McGregor JA, Allen KG, Harris MA, Reece M, Wheeler M, French JI, et al. The omega-3 story: nutritional prevention of preterm birth and other adverse pregnancy outcomes. Obstet Gynecol Surv. 2001;56(5 Suppl 1):S1–13.
• Meng Y, Groth SW. Fathers Count: The Impact of Paternal Risk Factors on Birth Outcomes. Matern Child Health J. 2018;22(3):401–8. https://doi.org/10.1007/s10995-017-2407-8 This is a retrospective cross-sectional analysis of birth certificate records (2004 to 2015) from the Finger Lakes Region in New York. Potential paternal risk factors examined included age, race/ethnicity, and education on four birth outcomes (preterm birth, low birthweight, high birthweight and small for gestational age). After controlling for maternal factors, several paternal factors (age, race, education) were found to contribute significantly to birth outcomes.
Oldereid NB, Wennerholm UB, Pinborg A, Loft A, Laivuori H, Petzold M, et al. The effect of paternal factors on perinatal and paediatric outcomes: a systematic review and meta-analysis. Hum Reprod Update. 2018;24(3):320–89. https://doi.org/10.1093/humupd/dmy005.
Goisis A, Remes H, Barclay K, Martikainen P, Myrskyla M. Paternal age and the risk of low birth weight and preterm delivery: a Finnish register-based study. J Epidemiol Community Health. 2018;72(12):1104–9. https://doi.org/10.1136/jech-2017-210170.
Khandwala YS, Baker VL, Shaw GM, Stevenson DK, Lu Y, Eisenberg ML. Association of paternal age with perinatal outcomes between 2007 and 2016 in the United States: population based cohort study. BMJ. 2018;363:k4372. https://doi.org/10.1136/bmj.k4372.
Palomar L, DeFranco EA, Lee KA, Allsworth JE, Muglia LJ. Paternal race is a risk factor for preterm birth. Am J Obstet Gynecol. 2007;197(2):152 e1–7. https://doi.org/10.1016/j.ajog.2007.03.035.
Shachar BZ, Mayo JA, Lyell DJ, Stevenson DK, Shaw GM, Blumenfeld YJ. Risk for spontaneous preterm birth among inter-racial/ethnic couples. J Matern Fetal Neonatal Med. 2018;31(5):633–9. https://doi.org/10.1080/14767058.2017.1293029.
Simhan HN, Krohn MA. Paternal race and preterm birth. Am J Obstet Gynecol. 2008;198(6):644 e1–6. https://doi.org/10.1016/j.ajog.2007.11.046.
Li Y, Luo Z, Holzman C, Liu H, Margerison CE. Paternal race/ethnicity and risk of adverse birth outcomes in the United States, 1989-2013. AIMS Public Health. 2018;5(3):312–23. https://doi.org/10.3934/publichealth.2018.3.312.
Portha B, Grandjean V, Movassat J. Mother or father: who is in the front line? Mechanisms underlying the non-genomic transmission of obesity/diabetes via the maternal or the paternal line. Nutrients. 2019;11(2). https://doi.org/10.3390/nu11020233.
Dodd JM, Du Plessis LE, Deussen AR, Grivell RM, Yelland LN, Louise J, et al. Paternal obesity modifies the effect of an antenatal lifestyle intervention in women who are overweight or obese on newborn anthropometry. Sci Rep. 2017;7(1):1557. https://doi.org/10.1038/s41598-017-01672-w.
• Houfflyn S, Matthys C, Soubry A. Male Obesity: Epigenetic Origin and Effects in Sperm and Offspring. Curr Mol Biol Rep. 2017;3(4):288–96. https://doi.org/10.1007/s40610-017-0083-5 A number of recent studies have suggested or presented data indicating epigenetic changes in sperm can influence offspring health. This paper summarizes the current data on paternal obesity, molecular/epigenetic mechanisms impacting sperm and transmission of disease conditions to offspring. Perhaps of greater interest, they introduce the concept of “paternal origins of health and disease (POHaD)”.
Moss JL, Harris KM. Impact of maternal and paternal preconception health on birth outcomes using prospective couples' data in add health. Arch Gynecol Obstet. 2015;291(2):287–98. https://doi.org/10.1007/s00404-014-3521-0.
Galaviz-Hernandez C, Sosa-Macias M, Teran E, Garcia-Ortiz JE, Lazalde-Ramos BP. Paternal Determinants in Preeclampsia. Front Physiol. 2018;9:1870. https://doi.org/10.3389/fphys.2018.01870.
Guo L, Choufani S, Ferreira J, Smith A, Chitayat D, Shuman C, et al. Altered gene expression and methylation of the human chromosome 11 imprinted region in small for gestational age (SGA) placentae. Dev Biol. 2008;320(1):79–91. https://doi.org/10.1016/j.ydbio.2008.04.025.
Koukoura O, Sifakis S, Soufla G, Zaravinos A, Apostolidou S, Jones A, et al. Loss of imprinting and aberrant methylation of IGF2 in placentas from pregnancies complicated with fetal growth restriction. Int J Mol Med. 2011;28(4):481–7. https://doi.org/10.3892/ijmm.2011.754.
Koukoura O, Sifakis S, Zaravinos A, Apostolidou S, Jones A, Hajiioannou J, et al. Hypomethylation along with increased H19 expression in placentas from pregnancies complicated with fetal growth restriction. Placenta. 2011;32(1):51–7. https://doi.org/10.1016/j.placenta.2010.10.017.
He Y, Xie X, Tang W, Ma X. Maternal and paternal obesity and adverse pregnancy outcomes in China: a cohort study. Lancet. 2017. https://doi.org/10.1016/S0140-6736(17)33190-2.
Goran MI, Plows JF, Ventura EE. Effects of consuming sugars and alternative sweeteners during pregnancy on maternal and child health: evidence for a secondhand sugar effect. Proc Nutr Soc. 2018;1–10. https://doi.org/10.1017/S002966511800263X.
Quansah R, Jaakkola JJ. Paternal and maternal exposure to welding fumes and metal dusts or fumes and adverse pregnancy outcomes. Int Arch Occup Environ Health. 2009;82(4):529–37. https://doi.org/10.1007/s00420-008-0349-6.
Michalek JE, Rahe AJ, Boyle CA. Paternal dioxin, preterm birth, intrauterine growth retardation, and infant death. Epidemiology. 1998;9(2):161–7.
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378–455. https://doi.org/10.1210/er.2011-1050.
McCowan LM, North RA, Kho EM, Black MA, Chan EH, Dekker GA, et al. Paternal contribution to small for gestational age babies: a multicenter prospective study. Obesity (Silver Spring). 2011;19(5):1035–9. https://doi.org/10.1038/oby.2010.279.
Zhao L, Chen L, Yang T, Wang L, Wang T, Zhang S, et al. Parental smoking and the risk of congenital heart defects in offspring: an updated meta-analysis of observational studies. Eur J Prev Cardiol. 2019. https://doi.org/10.1177/2047487319831367.
Banderali G, Martelli A, Landi M, Moretti F, Betti F, Radaelli G, et al. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: a descriptive review. J Transl Med. 2015;13:327. https://doi.org/10.1186/s12967-015-0690-y.
Hillman S, Peebles DM, Williams DJ. Paternal metabolic and cardiovascular risk factors for fetal growth restriction: a case-control study. Diabetes Care. 2013;36(6):1675–80. https://doi.org/10.2337/dc12-1280.
Liu W, Huang C, Cai J, Wang X, Zou Z, Sun C. Household environmental exposures during gestation and birth outcomes: a cross-sectional study in Shanghai, China. Sci Total Environ. 2018;615:1110–8. https://doi.org/10.1016/j.scitotenv.2017.10.015.
Andriani H, Kuo HW. Adverse effects of parental smoking during pregnancy in urban and rural areas. BMC Pregnancy Childbirth. 2014;14:414. https://doi.org/10.1186/s12884-014-0414-y.
Cui H, Gong TT, Liu CX, Wu QJ. Associations between passive maternal smoking during pregnancy and preterm birth: evidence from a meta-analysis of observational studies. PLoS One. 2016;11(1):e0147848. https://doi.org/10.1371/journal.pone.0147848.
Wang L, Yang Y, Liu F, Yang A, Xu Q, Wang Q, et al. Paternal smoking and spontaneous abortion: a population-based retrospective cohort study among non-smoking women aged 20-49 years in rural China. J Epidemiol Community Health. 2018;72(9):783–9. https://doi.org/10.1136/jech-2017-210311.
Beszterda M, Franski R. Endocrine disruptor compounds in environment: as a danger for children health. Pediatr Endocrinol Diabetes Metab. 2018;24(2):88–95. https://doi.org/10.18544/PEDM-24.02.0107.
Gonzalez N, Marques M, Nadal M, Domingo JL. Occurrence of environmental pollutants in foodstuffs: a review of organic vs. conventional food. Food Chem Toxicol. 2019;125:370–5. https://doi.org/10.1016/j.fct.2019.01.021.
Zennegg M. Dioxins and PCBs in meat - still a matter of concern? Chimia (Aarau). 2018;72(10):690–6. https://doi.org/10.2533/chimia.2018.690.
Stillerman KP, Mattison DR, Giudice LC, Woodruff TJ. Environmental exposures and adverse pregnancy outcomes: a review of the science. Reprod Sci. 2008;15(7):631–50. https://doi.org/10.1177/1933719108322436.
Candela S, Bonvicini L, Ranzi A, Baldacchini F, Broccoli S, Cordioli M, et al. Exposure to emissions from municipal solid waste incinerators and miscarriages: a multisite study of the MONITER project. Environ Int. 2015;78:51–60. https://doi.org/10.1016/j.envint.2014.12.008.
Hansen DA, Esakky P, Drury A, Lamb L, Moley KH. The aryl hydrocarbon receptor is important for proper seminiferous tubule architecture and sperm development in mice. Biol Reprod. 2014;90(1):8. https://doi.org/10.1095/biolreprod.113.108845.
Hernandez-Ochoa I, Karman BN, Flaws JA. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol. 2009;77(4):547–59. https://doi.org/10.1016/j.bcp.2008.09.037.
Lawrence BP, Vorderstrasse BA. New insights into the aryl hydrocarbon receptor as a modulator of host responses to infection. Semin Immunopathol. 2013;35(6):615–26. https://doi.org/10.1007/s00281-013-0395-3.
Mukerjee D. Health impact of polychlorinated dibenzo-p-dioxins: a critical review. J Air Waste Manag Assoc. 1998;48(2):157–65.
Emond C, Michalek JE, Birnbaum LS, DeVito MJ. Comparison of the use of a physiologically based pharmacokinetic model and a classical pharmacokinetic model for dioxin exposure assessments. Environ Health Perspect. 2005;113(12):1666–8. https://doi.org/10.1289/ehp.8016.
White SS, Birnbaum LS. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(4):197–211. https://doi.org/10.1080/10590500903310047.
Pesatori AC, Consonni D, Bachetti S, Zocchetti C, Bonzini M, Baccarelli A, et al. Short- and long-term morbidity and mortality in the population exposed to dioxin after the "Seveso accident". Ind Health. 2003;41(3):127–38.
Eskenazi B, Warner M, Brambilla P, Signorini S, Ames J, Mocarelli P. The Seveso accident: a look at 40years of health research and beyond. Environ Int. 2018;121(Pt 1:71–84. https://doi.org/10.1016/j.envint.2018.08.051.
Mocarelli P, Gerthoux PM, Needham LL, Patterson DG Jr, Limonta G, Falbo R, et al. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect. 2011;119(5):713–8. https://doi.org/10.1289/ehp.1002134.
Mocarelli P, Gerthoux PM, Patterson DG Jr, Milani S, Limonta G, Bertona M, et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116(1):70–7. https://doi.org/10.1289/ehp.10399.
Aurell J, Gullett BK, Yamamoto D. Emissions from open burning of simulated military waste from forward operating bases. Environ Sci Technol. 2012;46(20):11004–12. https://doi.org/10.1021/es303131k.
Doucet I. Desert storm syndrome: sick soldiers and dead children? Med War. 1994;10(3):183–94.
Emmerova M, Jirava F. Is gulf War syndrome really a mystery? Med Confl Surviv. 2004;20(3):209–17. https://doi.org/10.1080/1362369042000248811.
Woodall BD, Yamamoto DP, Gullett BK, Touati A. Emissions from small-scale burns of simulated deployed U.S. military waste. Environ Sci Technol. 2012;46(20):10997–1003. https://doi.org/10.1021/es3021556.
Butler DA, Styka AN, Savitz DA, editors. Assessment of the Department of Veterans Affairs Airborne Hazards and Open Burn Pit Registry. Washington (DC); 2017.
Lewis J. Smokey Bear in Vietnam. Environ Hist. 2006;11(3):598–603.
Institute Of Medicine. Veterans and agent Orange: health effects of herbicides used in Vietnam. Washington, DC: National Academies Press; 1994.
Ornstein C, Fresques H. ProPublica, Hixenbaugh M. The children of agent Orange. Virginia-pilot. 2016. https://www.propublica.org/article/the-children-of-agent-orange. 23 May 19
Peterson B. Vietnam War veterans' kids say agent Orange impact 'a nightmare'. 2018. https://abcnews.go.com/Politics/vietnam-war-veterans-kids-agent-orange-impact-nightmare/story?id=59059570. 23 May 19
WFLA. Agent Orange effects being seen in grandchildren of Vietnam War veterans. 2018. https://www.wfla.com/8-on-your-side/investigations/impacts-being-seen-in-grandchildren-of-vietnam-war-veterans-is-it-agent-orange-/1183887612. 23 May 19
Part 2: Making women and men. In Johnson M, editor. Essential reproduction. Wiley; 2010.
Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol. 2008;25(1):2–6. https://doi.org/10.1016/j.reprotox.2007.09.001.
U.S. War Dog Association. Vietnam Statistics. http://www.uswardogs.org/vietnam-statistics/. 15 April 19
Stellman JM, Stellman SD. Agent Orange during the Vietnam War: the lingering issue of its civilian and military health impact. Am J Public Health. 2018;108(6):726–8. https://doi.org/10.2105/AJPH.2018.304426.
Anh NT, Nishijo M, Tai PT, Maruzeni S, Morikawa Y, Anh TH, et al. Maternal risk factors associated with increased dioxin concentrations in breast milk in a hot spot of dioxin contamination in Vietnam. J Expo Sci Environ Epidemiol. 2014;24(5):489–96. https://doi.org/10.1038/jes.2013.73.
Nghi TN, Nishijo M, Manh HD, Tai PT, Van Luong H, Anh TH, et al. Dioxins and Nonortho PCBs in breast Milk of Vietnamese mothers living in the largest hot spot of dioxin contamination. Environ Sci Technol. 2015;49(9):5732–42. https://doi.org/10.1021/es506211p.
Schecter A, Pavuk M, Constable JD, Daile C, Papke O. A follow-up: high level of dioxin contamination in Vietnamese from agent orange, three decades after the end of spraying. J Occup Environ Med. 2002;44(3):218–20.
Tai PT, Nishijo M, Anh NT, Maruzeni S, Nakagawa H, Van Luong H, et al. Dioxin exposure in breast milk and infant neurodevelopment in Vietnam. Occup Environ Med. 2013;70(9):656–62. https://doi.org/10.1136/oemed-2012-101021.
Ngo TH, Hien TT, Thuan NT, Minh NH, Chi KH. Atmospheric PCDD/F concentration and source apportionment in typical rural, agent Orange hotspots, and industrial areas in Vietnam. Chemosphere. 2017;182:647–55. https://doi.org/10.1016/j.chemosphere.2017.05.050.
Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31(3):344–50. https://doi.org/10.1016/j.reprotox.2010.10.003.
Bruner-Tran KL, Ding T, Yeoman KB, Archibong A, Arosh JA, Osteen KG. Developmental exposure of mice to dioxin promotes transgenerational testicular inflammation and an increased risk of preterm birth in unexposed mating partners. PLoS One. 2014;9(8):e105084. https://doi.org/10.1371/journal.pone.0105084.
McConaha ME, Ding T, Lucas JA, Arosh JA, Osteen KG, Bruner-Tran KL. Preconception omega-3 fatty acid supplementation of adult male mice with a history of developmental 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure prevents preterm birth in unexposed female partners. Reproduction. 2011;142(2):235–41. https://doi.org/10.1530/REP-11-0070.
Zeng Y, Chen T. DNA methylation reprogramming during mammalian development. Genes (Basel). 2019;10(4). https://doi.org/10.3390/genes10040257.
Koutsaki M, Sifakis S, Zaravinos A, Koutroulakis D, Koukoura O, Spandidos DA. Decreased placental expression of hPGH, IGF-I and IGFBP-1 in pregnancies complicated by fetal growth restriction. Growth Hormon IGF Res. 2011;21(1):31–6. https://doi.org/10.1016/j.ghir.2010.12.002.
Kadakia R, Josefson J. The relationship of insulin-like growth factor 2 to fetal growth and adiposity. Horm Res Paediatr. 2016;85(2):75–82. https://doi.org/10.1159/000443500.
Rogero MM, Calder PC. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. 2018;10(4). https://doi.org/10.3390/nu10040432.
Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75(3):645–62. https://doi.org/10.1111/j.1365-2125.2012.04374.x.
Massiera F, Barbry P, Guesnet P, Joly A, Luquet S, Moreilhon-Brest C, et al. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J Lipid Res. 2010;51(8):2352–61. https://doi.org/10.1194/jlr.M006866.
Hadley EE, Richardson LS, Torloni MR, Menon R. Gestational tissue inflammatory biomarkers at term labor: a systematic review of literature. Am J Reprod Immunol. 2018;79(2):e12776. https://doi.org/10.1111/aji.12776.
Burris HH, Baccarelli AA, Wright RO, Wright RJ. Epigenetics: linking social and environmental exposures to preterm birth. Pediatr Res. 2016;79(1–2):136–40. https://doi.org/10.1038/pr.2015.191.
Nieuwenhuijsen MJ, Dadvand P, Grellier J, Martinez D, Vrijheid M. Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environ Health. 2013;12:6. https://doi.org/10.1186/1476-069X-12-6.
Porpora MG, Piacenti I, Scaramuzzino S, Masciullo L, Rech F, Benedetti Panici P. Environmental contaminants exposure and preterm birth: a systematic review. Toxics. 2019;7(1). https://doi.org/10.3390/toxics7010011.
Kumar S, Sharma S, Thaker R. Occupational, environmental, and lifestyle factors and their contribution to preterm birth - an overview. Indian J Occup Environ Med. 2017;21(1):9–17. https://doi.org/10.4103/ijoem.IJOEM_155_16.
Acknowledgments
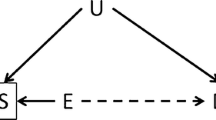
We gratefully acknowledge the assistance of Ms. Evelyn Hipp for contributing her artistic talent to Fig. 1.
Funding
Studies presented herein were supported in part by VA I01 BX002853, NIEHS ES14942, Amag Pharmaceuticals and the Vanderbilt University School of Medicine Medical Scholars Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shilpa Mokshagundam, Alison Barlow, and Tianbing Ding declare no conflict of interest. Kaylon L. Bruner-Tran reports grants from National Institute of Environmental Health Science, the Department of Veteran Affairs, the Environmental Protection Agency, and from AMAG Pharmacueticals, during the conduct of the study. Kevin G. Osteen reports grants from Gates Foundation, the Environmental Protection Agency, the National Institute of Environmental Health Science, and from the Department of Veteran Affairs, during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any new studies with humans or animals subjects performed by the any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Environmental Exposures and Pregnancy Outcomes
Rights and permissions
About this article
Cite this article
Bruner-Tran, K.L., Mokshagundam, S., Barlow, A. et al. Paternal Environmental Toxicant Exposure and Risk of Adverse Pregnancy Outcomes. Curr Obstet Gynecol Rep 8, 103–113 (2019). https://doi.org/10.1007/s13669-019-00265-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-019-00265-w