Abstract
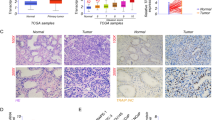
S100A16 is a member of the S100 calcium-binding protein family. It is overexpressed in many types of tumors and associated with proliferation, migration, and invasion; however, its function in human prostate cancer is unresolved. Our objective was to determine its effects and the underlying pathways of S100A16 in prostate cancer tissues and cells. We measured S100A16 expression by quantitative real-time polymerase and Western blotting in eight matched prostate cancer and adjacent normal tissues, and in three prostate cancer cell lines, DU-145, LNCaP, and PC-3, compared to a normal prostate epithelial cell line PrEC. DU-145 cells stably overexpressing S100A16 and PC-3 cells with S100A16 knockdown were established by transfection with S100A16 overexpression plasmid or shRNAs. Invasion, migration, and proliferation were analyzed by transwell assay, wound healing, and colony formation assays, respectively. Western blotting and invasion assays were performed to determine expressions and activation of AKT, ERK, p21, and p27. S100A16 was significantly overexpressed in both prostate cancer tissues and cells lines compared to normal controls (P < 0.05). Overexpression of S100A16 significantly promoted invasion, migration, and proliferation in prostate cancer cells in vitro, whereas silencing S100A16 showed the converse effects (P < 0.05). Furthermore, overexpression of S100A16 activated cell signaling proteins AKT and ERK and downregulated tumor suppressors p21 and p27. Specific inhibitors, LY294002 and PD98059, suppressed activation of AKT and ERK, which attenuated DU-145 cell clone formation and invasion induced by S100A16 overexpression. S100A16 may promote human prostate cancer progression via signaling pathways involving AKT, ERK, p21, and p27 downstream effectors. Our findings suggest that S100A16 may serve as a novel therapeutic or diagnostic target in human prostate cancer.






Similar content being viewed by others
References
Kiess AP, Cho SY, Pomper MG. Translational molecular imaging of prostate cancer. Curr Radiol Rep. 2013;1(3):216–26.
Sturchler E, Cox JA, Durussel I, Weibel M, Heizmann CW. S100A16, a novel calcium-binding protein of the EF-hand superfamily. J Biol Chem. 2006;281:38905–17.
Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450(3):191–231.
Heizmann CW. The multifunctional S100 protein family. Methods Mol Biol. 2002;172:69–80.
Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, et al. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. PNAS. 2006;103(40):14825–30.
Marenholz I, Heizmann CW. S100A16, a ubiquitously expressed EF-hand protein which is up-regulated in tumors. Biochem Biophys Res Commun. 2004;313:237–44.
Liu Y, Zhang R, Xin J, Sun Y, Li J, Wei D, et al. Identification of S100A16 as a novel adipogenesis promoting factor in 3T3-L1 cells. Endocrinology. 2011;152:903–11.
Liu H, Wang L, Wang X, Cao Z, Yang Q, Zhang K. S100A7 enhances invasion of human breast cancer MDA-MB-468 cells through activation of nuclear factor-κB signaling. World J Surg Oncol. 2013;11(93):1–8.
Saleem M, Adhami VM, Ahmad N, Gupta S, Mukhtar H. Prognostic significance of metastasis-associated protein S100A4 (Mts1) in prostate cancer progression and chemoprevention regimens in an autochthonous mouse model. Clin Cancer Res. 2005;11:147–53.
Yao R, Lopez-Beltran A, Maclennan GT, Montironi R, Eble JN, Cheng L. Expression of S100 protein family members in the pathogenesis of bladder tumors. Anticancer Res. 2007;27:3051–8.
Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. EJSO. 2008;34:357–64.
Hermani A, Hess J, De Servi B, Medunjanin S, Grobholz R, Trojan L, et al. Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res. 2011;11(14):5146–52.
Aalinkeel R, Nair MP, Sufrin G, Mahajan SD, Chadha KC, Chawda RP, et al. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004;64:5311–21.
Grotterød I, Maelandsmo GM, Boye K. Signal transduction mechanisms involved in S100A4-induced activation of the transcription factor NF-κB. BMC Cancer. 2010;10:241.
Grigorian M, Andresen S, Tulchinsky E, et al. Tumor suppressor p53 protein is a new target for the metastasis-associated Mts1/S100A4 protein: functional consequences of their interaction. J Biol Chem. 2001;276:22699–708.
Ginzburg S, Golovine KV, Makhov PB, Uzzo RG, Kutikov A, Kolenko VM. Piperlongumine inhibits NF-κB activity and attenuates aggressive growth characteristics of prostate cancer cells. Prostate. 2013. doi:10.1002/pros.22739.
Stearns M, Stearns ME. Evidence for increased activated metalloproteinase 2 (MMP-2a) expression associated with human prostate cancer progression. Oncol Res. 1996;8:69–75.
Price DJ, Avraham S, Feuerstein J, Fu Y, Avraham HK. The invasive phenotype in HMT-3522 cells requires increased EGF receptor signaling through both PI 3-kinase and ERK 1,2 pathways. Cell Commun Adhes. 2002;9(2):87–102.
Peng S, Zhang Y, Zhang J, Wang H, Ren B. ERK in learning and memory: a review of recent research. Int J Mol Sci. 2010;11(1):222–32.
Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22(4):954–65.
Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405.
Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18(1):77–82.
Dixon KM, Lui GY, Kovacevic Z, Zhang D, Yao M, Chen Z, Dong Q, Assinder SJ, Richardson DR., Dp44mT targets the AKT, TGF-b and ERK pathways via the metastasis suppressor NDRG1 in normal prostate epithelial cells and prostate cancer cells. Br J Cancer, 2013: 1–11.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81270952) and the Jiangsu Province’s Key Provincial Talents Program (RC201169), the Nanjing Medical Science and Technology Program (ZKX12017), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Supported by the Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU (No. 20113012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Weidong Zhu, Yi Xue and Chao Liang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, W., Xue, Y., Liang, C. et al. S100A16 promotes cell proliferation and metastasis via AKT and ERK cell signaling pathways in human prostate cancer. Tumor Biol. 37, 12241–12250 (2016). https://doi.org/10.1007/s13277-016-5096-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5096-9