Abstract
Introduction
Recent evidence from histology studies regarding random prostate biopsies hint toward a relationship between higher biopsy Gleason score and the development of metastatic castration resistant prostate cancer (mCRPC). However, prostate biopsy underestimates final pathology in about one-third of patients. We evaluated the final whole gland pathology from radical prostatectomy exclusively in order to assess the true risk of progressing to the mCRPC state for patients with confirmed Gleason ≤6 prostate cancer.
Methods
Patients with confirmed mCRPC from our outpatient clinic were retrospectively evaluated with regard to whole gland pathology and the occurrence of Gleason 6 histology from 1995 to 2015. Conversely, patients with confirmed Gleason 6 pathology from our institutional database were followed up for the development of mCRPC from 2001 to 2015. Kaplan–Meier analysis and the log rank test were applied for survival analysis. The binomial test was used to evaluate occurrence rates of Gleason ≤6 pathologies in mCRPC patients.
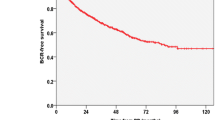
Results
Out of 62 patients with mCRPC none had confirmed Gleason 6 pathology on whole gland histology of the prostate. Out of 86 patients with confirmed Gleason 6 pathology none developed an mCRPC over the follow-up period.
Conclusion
The development of mCRPC in patients with true Gleason 6 pathology is very rare and could not be confirmed in our series. This finding may have important implications in future treatment planning.








Similar content being viewed by others
References
Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48.
Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Mellinger GT, Gleason D, Bailar J 3rd. The histology and prognosis of prostatic cancer. J Urol. 1967;97:331–7.
Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64.
Delahunt B, Egevad L, Grignon DJ, Srigley JR, Samaratunga H. Prostate cancer grading: recent developments and future directions. BJU Int. 2016;117(Suppl 4):7–8.
Corcoran NM, Hong MKH, Casey RG, et al. Upgrade in Gleason score between prostate biopsies and pathology following radical prostatectomy significantly impacts upon the risk of biochemical recurrence. BJU Int. 2011;108(8 Pt 2):E202–10.
Da Rosa MR, Milot L, Sugar L, et al. A prospective comparison of mRI-uS fused targeted biopsy versus systematic ultrasound-guided biopsy for detecting clinically significant prostate cancer in patients on active surveillance. J Magn Reson Imaging. 2015;41:220–5.
R Core Team. R: a language and environment for statistical computing. Vienna: R foundation for statistical computing; 2014. http://www.R-project.org/.
Busch J, Stephan C, Herold A, et al. Long-term oncological and continence outcomes after laparoscopic radical prostatectomy: a single-centre experience. BJU Int. 2012;110(11 Pt C):E985–90.
Dorin RP, Daneshmand S, Lassoff MA, Cai J, Skinner DG, Lieskovsky G. Long-term outcomes of open radical retropubic prostatectomy for clinically localized prostate cancer in the prostate-specific antigen era. Urology. 2012;79:626–31.
Lopez-Beltran A, Cheng L, Blanca A, Montironi R. Cell proliferation and apoptosis in prostate needle biopsies with adenocarcinoma Gleason score 6 or 7. Anal Quant Cytol Histol. 2012;34:61–5.
Delgado-Cruzata L, Hruby GW, Gonzalez K, et al. DNA methylation changes correlate with Gleason score and tumor stage in prostate cancer. DNA Cell Biol. 2012;31:187–92.
Nakabayashi M, Hayes J, Taplin M-E, et al. Clinical predictors of survival in men with castration-resistant prostate cancer: evidence that Gleason score 6 cancer can evolve to lethal disease. Cancer. 2013;119:2990–8.
van Soest RJ, de Morrée ES, Shen L, Tannock IF, Eisenberger MA, de Wit R. Initial biopsy Gleason score as a predictive marker for survival benefit in patients with castration-resistant prostate cancer treated with docetaxel: data from the TAX327 study. Eur Urol. 2014;66:330–6.
Patrikidou A, Loriot Y, Eymard J-C, et al. Who dies from prostate cancer? Prostate Cancer Prostatic Dis. 2014;17:348–52.
Schröder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–90.
Chowdhury S, Robinson D, Cahill D, Rodriguez-Vida A, Holmberg L, Møller H. Causes of death in men with prostate cancer: an analysis of 50,000 men from the Thames Cancer Registry. BJU Int. 2013;112:182–9.
Ang M, Rajcic B, Foreman D, Moretti K, O’Callaghan ME. Men presenting with prostate-specific antigen (pSA) values of over 100 ng/mL. BJU Int. 2016;117(Suppl 4):68–75.
Pinsky P, Parnes H, Ford L. Estimating rates of true high-grade disease in the prostate cancer prevention trial. Cancer Prev Res (Phila). 2008;1:182–6.
Danneman D, Drevin L, Robinson D, Stattin P, Egevad L. Gleason inflation 1998–2011: a registry study of 97,168 men. BJU Int. 2015;115:248–55.
Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL, ISUP Grading Committee. The International Society of Urological Pathology (iSUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;2005(29):1228–42.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Alena Böker and Katharina Krüger have nothing to disclose. Markus A. Kuczyk: Honoraria and speaker for Astellas, Bayer, BMS, Eisai, Janssen, Novartis, Pfizer, and Pierre Fabre. Advisor for BMS, Eisai, Farco-Pharma, Pfizer, and Storz. Mario W. Kramer: Honoraria and speaker for Astellas, Bayer, Pierre Fabre, Eisai, Roche, Pfizer, Sanofi, and Novartis. Axel S. Merseburger: Honoraria and speaker for Amgen, Astellas, Bayer, Ferring, Hexal, Ipsen, Janssen, Novartis, Roche, TEVA, and Sanofi. Florian Imkamp: Honoraria and travel grants from Baxter, Astellas, Pfizer, Novartis, and Pierre Fabre. Christoph-A. von Klot: Honoraria and speaker for Janssen, Astellas, Bayer, BMS, Ipsen, Novartis, and TEVA.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors. The authors acknowledge Marina Akkermann, Nadja Bergen, and Gerd Wegener for excellent patient and database assistance.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available since some individual therapeutic and clinical data may identify patients in a relatively small series from a single institute but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/F227F0600CA7BBCF.
Rights and permissions
About this article
Cite this article
Böker, A., Kuczyk, M.A., Kramer, M.W. et al. True Incidence of Gleason 6 Pathology in Patients with Metastatic Castration Resistant Prostate Cancer (mCRPC). Adv Ther 34, 171–179 (2017). https://doi.org/10.1007/s12325-016-0450-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-016-0450-2