Abstract
The aim of this study was to determine the impact of ABCB1 polymorphism, BMI, age and drug co-administration on safety and efficiency of posaconazole (PCZ) oral suspension treatment in children with hematological diseases. Seventy children were included in the study. ABCB1 polymorphism in fifty-eight children was determined using a PCR–RFLP method. A protocol with data on the health condition, treatment and adverse events (AE), as well as a survey on treatment tolerance for the legal guardians was evaluated. Liver function tests were observed for the first 20 days, and AE during the complete medication period. For statistical analysis a χ2 test with Yates’s correction, a Pearson’s or Spearman’s correlation test was performed (p < 0.05). Genetic testing showed 24% CC, 46% CT and 30% of TT variants. PCZ prophylaxis failed in twenty cases, where change in prophylactic treatment was needed. Fifty-two children suffered from at least one mild to moderate adverse event. Sixty-five legal guardians completed the survey, most of them reported the treatment to be well tolerated. ABCB1 polymorphism had no impact on AE occurrence and posaconazole prophylaxis efficiency. Age influenced the number of gastrointestinal (p = 0.02), visual (p = 0.05), neurological (p = 0.01), dermatological (p = 0.002) and flu-like (p = 0.02) complications. AST (p = 0.03) and LDH (p = 0.008) activity presented age dependency. The concomitant use of proton pump inhibitors (PPI) had impact on liver health parameters elevation (p = 0.009) and circulatory system complications (p = 0.008). High incidence of mild to moderate AE, and other factors influencing PCZ pharmacokinetics (PPI co-administration, obesity), suggest a need for careful pediatric onco-hematology patient evaluation.
Similar content being viewed by others
Introduction
Posaconazole (PCZ) is a triazole antifungal agent. The drug is primarily indicated for molds infection prophylaxis in patients receiving chemotherapy for acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and in patients after allogenic hematopoietic stem cell transplantation (allo-HSCT).
It may also be used for treatment of particular cases of invasive aspergillosis, fusariosis, chromoblastomycosis, coccidioidomycosis and candidiasis [1, 2].
The treatment of children is very specific. Not only from the psychological point of view, also the pharmacokinetic properties of drugs differ from the adult population. Basic alterations are gathered in Table 1 [3, 4].
According to the Food and Drug Administration (FDA) PCZ oral formulation is approved for children older than 13 [2, 3]. Some studies showed that the agent is also safe in children younger than 12 [5]. Although posaconazole has either no grading (in cases of children after allo-HSCT without GVHD) or a BI grade (in children after allo-HSCT with GVHD and in de novo or recurrent leukemias) according to the Recommendations for primary chemoprophylaxis of invasive fungal diseases in paediatric patients with cancer or haemopoietic stem-cell transplantation of the ECIL-4, it is often used in clinic for antifungal prophylaxis in children with hematological malignances [6].
The pediatric onco-hematology population is particularly exposed to adverse drug reactions (ADR) and adverse events (AE). The effectiveness and safety of PCZ oral suspension is influenced mainly by food intake, gastric pH and motility as well as drug-drug interactions [5, 7, 8]. Posaconazole is a substrate for UDP-glucuronosyltransferase and P-glycoprotein, thus agents having impact on these two clearance pathways must be taken into consideration during PCZ medication [1].
As P-glycoprotein is encoded by the ABCB1 gene, the polymorphism of which is connected with changes in the protein expression, the aim of our study was to determine the impact of ABCB1 polymorphism on the safety and efficiency of posaconazole oral suspension treatment in children. We also took into consideration other agents potentially influencing antifungal prophylaxis (BMI, age, drug co-administration). To our knowledge it is the first of this kind study performed among the Polish pediatric population with hematological diseases.
Materials and Methods
The study was performed according to the Declaration of Helsinki and with approval of the Bioethics Committee of the Wrocław Medical University (KB-657/2012). It is a single center, non-randomized retrospective analysis of seventy pediatric patients who received posaconazole oral suspension for antifungal prophylaxis.
Patients Characteristics
The included children were patients of the Paediatric Bone Marrow Transplantation, Oncology and Hematology Unit, of the Wrocław Medical University Hospital, aged between 2 months and 19 years. The median PCZ dose was 8 mg per kg of body weight, two times a day according to the algorithm evaluated by Welzen et al. [9]. Most important data on patients enrolled is presented in Table 2.
Patients’ Protocol and Survey
A protocol with most important data on patients health condition, underlying diseases, allo-HSCT performance, previous infections, treatment and adverse events was evaluated. As posaconazole may lead to hepatic impairment we also monitored biochemical parameters of liver function. Those were aspartate aminotransferase (AST), alanine transaminase (ALT), activated partial thromboplastin time (APTT), gamma-glutamyltransferase (GGT), lactate dehydrogenase (LDH) activity, albumin and fibrinogen concentrations at three time points (before, at the seventh, and the twentieth day) of PCZ prophylaxis.
Drug-drug interactions are also factors influencing PCZ pharmacokinetics and inducing AE occurrence. So, we tried to determine, on the basis of patients treatment history, drugs potentially interacting with the antifungal agent. We monitored AE during the complete posaconazole conducted medication period.
In addition a survey on treatment tolerance and observed adverse events was evaluated and performed among the legal guardians of the examined children. Sixty-five of them completed the questionnaire.
The survey consisted of five parts: first—connected with basic information (body weight (BW), age, height), second—with information on previous fungal infections (occurred before hospital admission), third—potential food supplement administration, fourth—adverse events observed during posaconazole treatment (we intentionally listed AE possibly occurring during PCZ therapy) and fifth—concerning the treatment satisfaction in a 0–5 score scale. On this scale 0 meant no opinion, 1-no satisfaction (serious fungal infection occurred despite posaconazole prophylaxis), 2-low satisfaction (local fungal infection in more than one body area occurred), 3-moderate satisfaction (local fungal infection occurred), 4-satisfaction (no fungal infection, but adverse events occurred), 5-full satisfaction (no fungal infection and good tolerance).
DNA Isolation and Genetic Testing
We collected seventy samples (one from each patient) of whole blood drawn on EDTA. The samples were stored at -20 °C.
DNA was isolated using the QIAamp® DNA Blood Mini Kit according to the manufacturer instruction, in a laminar flow cabinet.
For ABCB1 polymorphism determination, an adapted Siegmund et al. [10] polymerase chain reaction-restriction length polymorphism (PCR–RFLP) method was used.
Statistical Analysis
We performed statistical analysis on the collected data. For every group the number of cases (N), median (M), range (min–max), upper and lower quartile (25Q-75Q) was calculated. The hypothesis on the equality of median values of the groups was verified using the Kruskal–Wallis test.
For discrete parameters the frequency of features in the groups was analyzed using the Chi squared test with Yates’s correction, with an adequate number of degrees of freedom df (df = (m − 1) * (n − 1)).
For chosen pairs of parameters a Pearson’s or Spearman’s correlation test was performed.
p values less than 0.05 were considered statistically significant. p values between 0.05 and 0.1 were considered as tendency. The statistical analysis was performed using the EPIINFO program (Ver. 7.1.1.14, 2013, USA).
Results
Seventy patients of the Paediatric Bone Marrow Transplantation, Oncology and Hematology Unit, of the Wrocław Medical University Hospital were included in our study. The median BMI was 16.4 kg/m2, two children were overweight. During the observation period 21 (30%) children suffered from acute GVHD, seven of them further developed a chronic form of the disease.
Fifty-eight legal guardians agreed for genetic testing of their children. The performed C3435T genetic testing for ABCB1 polymorphisms showed that 24% (N = 14) of patients presented the CC, 46% (N = 27) the CT, and 30% (N = 17) the TT variant.
Posaconazole oral formulation prophylaxis failed in 20 cases, where a change in treatment was needed according to suspected IFI development.
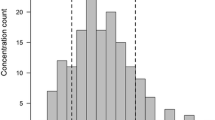
Fifty-two children suffered from at least one mild to moderate adverse event during invasive fungal infection prophylactic treatment. However according to the performed survey legal guardians reported the treatment to be well tolerated by their children (number of completed surveys 65; score 5 N = 21, score 4 N = 21, score 3 N = 10, score 2 N = 2, score 1 N = 0, score 0 N = 11). There was no need to discontinue posaconazole prophylaxis due to adverse events in any case. The occurrence of AE among different systems is presented in Fig. 1.
Increased AST activity was observed in 35 cases, ALT activity increased in 42, GGT in 28 cases. Nine children presented higher bilirubin values. Most frequent gastrointestinal disturbances were vomiting (N = 29), nausea (N = 25), loss of appetite (N = 22), diarrhea (N = 21), constipation (N = 20) and abdominal pain (N = 20). Dermatological complication manifested as mouth ulceration (N = 24) and rash (N = 11). Nervous system disorders showed as headache (N = 10), insomnia (N = 7) and tremor (N = 5). Ophthalmological disorders most often were connected with blurred vision (N = 9) whereas cardiovascular disorders with hypertension (N = 6). Renal disorders manifested as blood urea concentration increase in 5 and hemorrhagic cystitis in 2 cases.
Drug-drug interaction analysis led to determination of agents potentially inducing AE occurrence during PCZ treatment, having impact on its pharmacokinetic properties. Drugs and number of children treated are presented in Table 3.
Statistical analysis showed that ABCB1 polymorphism has no impact on overall adverse event occurrence and posaconazole prophylaxis efficiency as presented in Table 4. However two patients with good tolerance to posaconazole, who did not suffer from any adverse event during antifungal treatment presented the CC variant. We found a tendency for APTT prolongation during treatment in individuals presenting the TT variant (p = 0.07, χ2 = 8.45). After twenty days of posaconazole treatment the APTT prolonged in four patients presenting the CT variant (N = 27, 14.8%), one patient with CC variant (N = 14, 0.07%) and in 7 patients presenting the TT variant (N = 17, 41.2%).
The age of children influenced the number of gastrointestinal, visual, neurological, dermatological and flu-like complications. The older the child the more often mentioned AE occurred. Furthermore AST and LDH activity presented age dependency especially in the first week of PCZ treatment.
Children with higher BMI values were more susceptible for visual and skin disturbances (p = 0.057, H = 3.61) as well as increased LDH activity during the first week of IFI prophylaxis.
Gender did not influence AE frequency.
The concomitant use of proton pump inhibitors (PPI) had great impact on parameters for liver health elevation and circulatory system complications during PCZ prophylaxis.
Most important findings on variables influencing adverse events during posaconazole conducted prophylaxis and their statistical significance are presented in Table 5.
Discussion
The aim of this investigation was to determine the impact of ABCB1 polymorphism, BMI, age and drug co-administration on safety and efficiency of posaconazole oral suspension treatment in children with hematological diseases.
In our study the frequencies of ABCB1 C3435T genetic variants (24% for CC, 46% for CT and 30% for the TT variant) were comparable with other research performed among the Polish hematology population [11, 12].
We evaluated the influence of ABCB1 polymorphism on adverse event occurrence in children with hematological malignances treated with PCZ oral suspension. We found no statistical correlation, however a tendency for APTT prolongation during treatment was observed. This finding is completely new and to our knowledge has not been observed in any research before.
What is also interesting in our study, is that patients with good tolerance to posaconazole and with proper prophylactic response (N = 2) presented the CC variant. This may be connected with higher activity of P-gp in patients with this ABCB1 genetic polymorphism, that was previously reported in patients with B cell chronic lymphocytic leukemia, and what comes along with the increased active transport of drugs out of cells, suggesting and supporting the thesis of a protective role of the C3435C variant [12].
We didn’t find a correlation between ABCB1 polymorphism and efficiency of PCZ prophylaxis measured as success or failure of prophylactic treatment. To our knowledge we were the first to evaluate this problem.
During posaconazole pharmacotherapy relatively high incidence of mild to moderate adverse events occurred. Most frequent were gastrointestinal, visual, neurological, dermatological and flu-like complications. The observations are concise with those from other studies. Lehrnbecher et al. [13] reported fever, nausea and/or vomiting, abdominal pain, diarrhea, headache, and skin eruptions as most common during PCZ treatment. Gastrointestinal and skin adverse events, according the observations of Döring et al. [5], were the most common complications.
The elevation of liver function tests namely transaminase activity and LDH activity is a well-known adverse drug reaction caused by triazole antifungal agents [1, 5, 13,14,15]. What is a new finding, and as to our knowledge was not observed in any of the previous studies in children, is a statistically significant correlation between age and mentioned AE. The older the child the more often described adverse events occurred. A similar tendency was observed for adults, where a reduction in the apparent volume of distribution, and 11% higher average plasma posaconazole concentration were observed according to older age [16]. However as children generally have different pharmacokinetics than adults, this finding cannot be directly extrapolated into the pediatric population.
The concomitant use of proton pump inhibitors (omeprazole, pantoprazole) had impact on parameters for liver health and circulatory system complications during PCZ prophylaxis. The influence of concomitant PPI administration with posaconazole is well established for the adult population and was also evaluated in children [7, 16,17,18,19]. Although in general the PPI co-administration is connected with lower posaconazole serum concentrations, during our study we observed that adverse events occurred more often in patients receiving omeprazole or pantoprazole. This could be connected with other drugs used for hematological malignancy treatment. PPI—especially the older representatives (omeprazole, pantoprazole) are CYP2C19 substrates, and therefore might affect pharmacokinetics of other drugs metabolized through CYP2C19 [20].
We observed that children with higher BMI values are more susceptible for visual and skin disturbances as well as increased LDH activity during the first week of IFI prophylaxis conducted with posaconzole. This problem was not evaluated for the pediatric population receiving PCZ in oral suspension formulation. However there is one study describing the experience from using posaconazole in form of extended released tablets. In obese adult patients (BMI > 30 kg/m2, and body weight > 90 kg) the antifungal agents serum levels were lower during the tablet formulation treatment [21]. Our observations are not concise with this finding. This could be due to alterations of pharmacokinetic properties of both formulations and both populations (e.g. in children the glucuronidation potential is not as high as in adults, thus it cannot be increased in childhood obesity, as it is the case in the adult population).
Conclusion
ABCB1 polymorphism has no impact on the efficiency and safety of posaconazole treatment in children with hematological malignancies. However the relatively high incidence of mild to moderate adverse events during prophylaxis, and occurrence of other factors potentially influencing PCZ pharmacokinetics such as PPI co-administration or obesity, suggest a need for careful patient evaluation and probable TDM (therapeutic drug monitoring) enrollment in onco-hematological pediatric patients receiving antifungal prophylactic treatment conducted with posaconazole oral suspension formulation.
What is certainly a limitation of our study is the relatively small group of patients and lack of posaconazole serum concentration measurement. However the aim of our retrospective research was to evaluate potential causes of AE occurrence, and prophylaxis failure during PCZ treatment. As our findings were partially new there is certainly a need for larger studies to evaluate the problem in the future.
References
Summary of Product Characteristics Noxafil. Merck Sharp & Dohme Limited. 2010. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/000610/WC500037784.pdf. Accessed 3 Jul 2018
Noxafil (posaconazole)—package insert. Whitehouse Station, NJ: Merck & Co., Inc. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022003s018s020,0205053s002s004,0205596s001s003lbl.pdf. Accessed 3 Jul 2018
Lu H, Rosenbaum S (2014) Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther 19:262–276. https://doi.org/10.5863/1551-6776-19.4.262
Kodidela S, Kumar S, Uppugunduri CRS (2017) Developmental pattern of hepatic drug-metabolizing enzymes in pediatric population and its role in optimal drug treatment. Arch Med Health Sci 5:115–122
Döring M, Müller C, Johann PD, Erbacher A, Kimmig A, Schwarze CP, Lang P, Handgretinger R, Müller I (2012) Analysis of posaconazole as oral antifungal prophylaxis in pediatric patients under 12 years of age following allogeneic stem cell transplantation. BMC Infect Dis 12:263. https://doi.org/10.1186/1471-2334-12-263
Groll AH, Castagnola E, Cesaro S, Dalle JH, Engelhard D, Hope W, Roilides E, Styczynski J, Warris A, Lehrnbecher T et al (2014) Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol 15:327–340. https://doi.org/10.1016/s1470-2045(14)70017-8
Vicenzi EB, Calore E, Decembrino N, Berger M, Perruccio K, Carraro F, Rossin S, Putti MC, Molinaro M, Tridello G, Cesaro S (2018) Posaconazole oral dose and plasma levels in pediatric hematology–oncology patients. Eur J Haematol 100(3):315–322. https://doi.org/10.1111/ejh.13017
Döring M, Cabanillas Stanchi KM, Queudeville M, Feucht J, Blaeschke F, Schlegel P, Feuchtinger T, Lang P, Müller I, Handgretinger R, Heinz WJ (2017) Efficacy, safety and feasibility of antifungal prophylaxis with posaconazole tablet in paediatric patients after haematopoietic stem cell transplantation. J Cancer Res Clin Oncol 143:1281–1292. https://doi.org/10.1007/s00432-017-2369-7
Welzen ME, Brüggemann RJ, Van Den Berg JM, Voogt HW, Gilissen JH, Pajkrt D, Klein N, Burger DM, Warris A (2011) A twice daily posaconazole dosing algorithm for children with chronic granulomatous disease. Pediatr Infect Dis J 30(9):794–797. https://doi.org/10.1097/INF.0b013e3182195808
Siegmund W, Ludwig K, Giessmann T, Dazert P, Schroeder E, Sperker B et al (2002) The effects of the human MDR1 genotype on the expression of duodenal P-glycoprotein and disposition of the probe drug talinolol. Clin Pharmacol Ther 72:572–583
Jamroziak K, Młynarski W, Balcerczak E, Mistygacz M, Trelinska J, Mirowski M, Bodalski J, Robak T (2004) Functional C3435T polymorphism of MDR1 gene: an impact on genetic susceptibility and clinical outcome of childhood acute lymphoblastic leukemia. Eur J Hematol 72:314–321
Jamroziak K, Balcerczak E, Smolewski P, Robey RW, Cebula B, Panczyk M, Kowalczyk M, Szmigielska-Kapłon A, Mirowski M, Bates SE, Robak T (2006) MDR1 (ABCB1) gene polymorphism C3435T is associated with P-glycoprotein activity in B-cell chronic lymphocytic leukemia. Pharmacol Rep 58:720–728
Lehrnbecher T, Attarbaschi A, Duerken M, Garbino J, Gruhn B, Kontny U, Lüer S, Phillips R, Scholz J, Wagner HJ, Wiesel T, Groll AH (2010) Posaconazole salvage treatment in paediatric patients: a multicentre survey. Eur J Clin Microbiol Infect Dis 29:1043–1045. https://doi.org/10.1007/s10096-010-0957-4
Döring M, Blume O, Haufe S, Hartmann U, Kimmig A, Schwarze CP, Lang P, Handgretinger R, Müller I (2014) Comparison of itraconazole, voriconazole, and posaconazole as oral antifungal prophylaxis in pediatric patients following allogeneic hematopoietic stem cell transplantation. Eur J Clin Microbiol Infect Dis 33:629–638. https://doi.org/10.1007/s10096-013-1998-2
Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S (2007) Posaconazole or fluconazole for prophylaxis in severe graft-versus-host Disease. N Engl J Med. 356:335–347
Kohl V, Müller C, Cornely OA, Abduljalil K, Fuhr U, Vehreschild JJ, Scheid C, Hallek M, Rüping MJ (2010) Factors influencing pharmacokinetics of prophylactic posaconazole in patients undergoing allogeneic stem cell transplantation. Antimicrob Agents Chemother 54(1):207–212. https://doi.org/10.1128/AAC.01027-09
Li W, Zeng S, Yu LS, Zhou Q (2013) Pharmacokinetic drug interaction profile of omeprazole with adverse consequences and clinical risk management. Ther Clin Risk Manag 9:259–271. https://doi.org/10.2147/TCRM.S43151
Ross AL, Slain D, Cumpston A, Bryant AM, Hamadani M, Craig M (2012) Evaluation of an alternative posaconazole prophylaxis regimen in haematological malignancy patients receiving concomitant stress ulcer prophylaxis. Int J Antimicrob Agents 40:557–561. https://doi.org/10.1016/j.ijantimicag.2012.09.001
Bryant AM, Slain D, Cumpston A, Craig M (2011) A post-marketing evaluation of posaconazole plasma concentrations in neutropenic patients with haematological malignancy receiving posaconazole prophylaxis. Int J Antimicrob Agents 37:266–269. https://doi.org/10.1016/j.ijantimicag.2010.11.021
Gawrońska-Szklarz B, Adamiak-Giera U, Wyska E, Kurzawski M, Gornik W, Kaldonska M, Drozdzik M (2012) CYP2C19 polymorphism affects single-dose pharmacokinetics of oral pantoprazole in healthy volunteers. Eur J Clin Pharmacol 68:1267–1274. https://doi.org/10.1007/s00228-012-1252-3
Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR (2015) Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses 58:432–436. https://doi.org/10.1111/myc.12339
Acknowledgements
This study was founded by the Ministry of Science and Higher Education, Republic of Poland - the Wroclaw Medical University Grand for Young Scientists (STM.D120.16.035).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sienkiewicz-Oleszkiewicz, B., Urbańczyk, K., Stachowiak, M. et al. Factors Influencing the Safety and Efficiency of Antifungal Prophylaxis with Posaconazole in Children with Hematological Diseases: From Genetics to Polypharmacotherapy. Indian J Hematol Blood Transfus 35, 699–706 (2019). https://doi.org/10.1007/s12288-019-01134-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-019-01134-5