Abstract
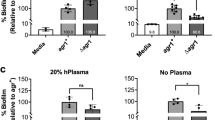
Staphylococci have quorum-sensing (QS) systems that enable cell-to-cell communication, as well as the regulation of numerous colonization and virulence factors. The accessory gene regulator (Agr) operon is one of the Staphylococcus genus QS systems. Three groups (I, II, and III) are present in Staphylococcus epidermidis Agr operon. To date, it is unknown whether Agr groups can interact symbiotically during biofilm development. This study analyzed a symbiotic association among Agr groups during biofilm formation in clinical and commensal isolates. Different combinations among Agr group isolates was used to study biofilm formation in vitro and in vivo (using a mouse catheter-infection model). The analysis of Agr groups were also performed from samples of human skin (head, armpits, and nostrils). Different predominant coexistence was found within biofilms, suggesting symbiosis type. In vitro, Agr I had a competition with Agr II and Agr III. Agr II had a competition with Agr III, and Agr II was an antagonist to Agr I and III when the three strains were combined. In vivo, Agr II had a competition to Agr I, but Agr I and II were antagonists to Agr III. The associations found in vitro and in vivo were also found in different sites of the skin. Besides, other associations were observed: Agr III antagonized Agr I and II, and Agr III competed with Agr I and Agr II. These results suggest that, in S. epidermidis, a symbiotic association of competition and antagonism occurs among different Agr groups during biofilm formation.
Similar content being viewed by others
References
Augustine, N., Kumar, P., and Thomas, S. 2010. Inhibition of Vibrio cholerae biofilm by AiiA enzyme produced from Bacillus spp. Arch. Microbiol. 192, 1019–1022.
Betanzos-Cabrera, G., Juárez-Verdayes, M.A., González-González, G., Cancino-Díaz, M.E., and Cancino-Díaz, J.C. 2009. Gatifloxacin, moxifloxacin, and balofloxacin resistance due to mutations in the gyrA and parC genes of Staphylococcus epidermidis strains isolated from patients with endophthalmitis, corneal ulcers and conjunctivitis. Ophthalmic Res. 42, 43–48.
Bijtenhoorn, P., Schipper, C., Hornung, C., Quitschau, M., Grond, S., Weiland, N., and Streit, W.R. 2011. BpiB05, a novel metagenome-derived hydrolase acting on N-acylhomoserine lactones. J. Biotechnol. 155, 86–94.
Canovas, J., Baldry, M., Bojer, M.S., Andersen, P.S., Grzeskowiak, P.K., Stegger, M., Damborg, P., Olsen, C.A., and Ingmer, H. 2016. Cross-talk between Staphylococcus aureus and other Staphylococcal species via the agr quorum sensing system. Front. Microbiol. 7, 1–13.
Chen, Y., Wang, X.Y., Huang, Y.C., Zhao, G.Q., Lei, Y.J., Ye, L.H., Huang, Q.B., and Duan, W.S. 2015. Study on the structure of Candida albicans-Staphylococcus epidermidis mixed species biofilm on polyvinyl chloride biomaterial. Cell Biochem. Biophys. 73, 461–468.
Christensen, G.D., Simpson, W.A., Younger, J.J., Baddour, L.M., Barrett, F.F., Melton, D.M., and Beachey, E.H. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22, 996–1006.
Chu, W., Zere, T.R., Weber, M.M., Wood, T.K., Whiteley, M., Hidalgo-Romano, B., Valenzuela, E. Jr., and McLean, R.J. 2012. Indole production promotes Escherichia coli mixed-culture growth with Pseudomonas aeruginosa by inhibiting quorum signaling. Appl. Environ. Microbiol. 78, 411–419.
Conlan, S., Mijares, L.A., Becker, J., Blakesley, R.W., Bouffard, G.G., Brooks, S., Coleman, H., Gupta, J., Gurson, N., Park, M., et al. 2012. Staphylococcus epidermidis pan-genome sequence analysis reveals diversity of skin commensal and hospital infection-associated isolates. Genome. Biol. 13, 1–13.
Dastgheyb, S.S., Villaruz, A.E., Le, K.Y., Tan, V.Y., Duong, A.C., Chatterjee, S.S., Cheung, G.Y., Joo, H.S., Hickok, N.J., and Otto, M. 2015. Role of phenol-soluble modulins in formation of Staphylococcus aureus biofilms in synovial fluid. Infect. Immun. 83, 2966–2975.
Davies, D.G., Parsek, M.R., Pearson, J.P., Iglewski, B.H., Costerton, J.W., and Greenberg, E.P. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280, 295–298.
de Araujo, G.L., Coelho, L.R., de Carvalho, C.B., Maciel, R.M., Coronado, A., Rozenbaum, R., Ferreira-Carvalho, B.T., Figueiredo, A.M., and Teixeira, L.A. 2006. Commensal isolates of methicillin-resistant Staphylococcus epidermidis are also well equipped to produce biofilm on polystyrene surfaces. J. Antimicrob. Chemother. 57, 855–864.
Dong, Y.H., Wang, L.H., and Zhang, L.H. 2007. Quorum-quenching microbial infections: mechanisms and implications. Phil. Trans. R. Soc. B. 362, 1201–1211.
Dufour, P., Jarraud, S., Vandenesch, F., Greenland, T., Novick, R.P., Bes, M., Etienne, J., and Lina, G. 2002. High genetic variability of the agr locus in Staphylococcus species. J. Bacteriol. 184, 1180–1186.
Duggirala, A., Kenchappa, P., Sharma, S., Peeters, J.K., Ahmed, N., Garg, P., Das, T., and Hasnain, S.E. 2007. High-resolution genome profiling differentiated Staphylococcus epidermidis isolated from patients with ocular infections and normal individuals. Investig. Ophthalmol. Vis. Sci. 48, 3239–3245.
Fuqua, C. and Greenberg, E.P. 2002. Listening in on bacteria: acylhomoserine lactone signalling. Nat. Rev. Mol. Cell. Biol. 3, 685–695.
Ghaznavi-Rad, E., Nor Shamsudin, M., Sekawi, Z., Van Belkum, A., and Neela, V. 2010. A simplified multiplex PCR assay for fast and easy discrimination of globally distributed staphylococcal cassette chromosome mec types in meticillin-resistant Staphylococcus aureus. J. Med. Microbiol. 59, 1135–1139.
Heilmann, C., Schweitzer, O., Gerke, C., Vanittanakom, N., Mack, D., and Götz, F. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20, 1083–1091.
Iwase, T., Uehara, Y., Shinji, H., Tajima, A., Seo, H., Takada, K., Agata, T., and Mizunoe, Y. 2010. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465, 346–349.
Janzon, L. and Arvidson, S. 1990. The role of the delta-lysin gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 9, 1391–1399.
Jarraud, S., Lyon, G.J., Figueiredo, A.M.S., Gerard, L., Vandenesch, F., Etienne, J., Muir, T.W., and Novick, R.P. 2000. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J. Bacteriol. 182, 6517–6522.
Jarraud, S., Mougel, C., Thioulouse, J., Lina, G., Meugnier, H., Forey, F., Nesme, X., Etienne, J., and Vandenesch, F. 2002. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 70, 631–641.
Jayathilake, P.G., Jana, S., Rushton, S., Swailes, D., Bridgens, B., Curtis, T., and Chen, J. 2017. Extracellular polymeric substance production and aggregated bacteria colonization influence the competition of microbes in biofilms. Front. Microbiol. 8, 1–14.
Ji, G., Beavis, R.C., and Novick, R.P. 1995. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. USA 92, 12055–12059.
Ji, G., Beavis, R., and Novick, R.P. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276, 2027–2030.
Juárez-Verdayes, M.A., Reyes-López, M.Á., Cancino-Díaz, M.E., Muñoz-Salas, S., Rodríguez-Martínez, S., Zavala-Díaz De La Serna, F.J., Hernández-Rodríguez, C.H., and Cancino-Díaz, J.C. 2006. Isolation, vancomycin resistance and biofilm production of Staphylococcus epidermidis from patients with conjunctivitis, corneal ulcers, and endophthalmitis. Rev. Latinoam. Microbiol. 48, 238–246.
Li, M., Guan, M., Jiang, X.F., Yuan, F.Y., Xu, M., Zhang, W.Z., and Lu, Y. 2004. Genetic polymorphism of the accessory gene regulator (agr) locus in Staphylococcus epidermidis and its association with pathogenicity. J. Med. Microbiol. 53, 545–549.
Lina, G., Jarraud, S., Ji, G., Greenland, T., Pedraza, A., Etienne, J., Novick, R.P., and Vandenesch, F. 1998. Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol. Microbiol. 28, 655–662.
Morfeldt, E., Panova-Sapundjieva, I., Gustafsson, B., and Arvidson, S. 1996a. Detection of the response regulator AgrA in the cytosolic fraction of Staphylococcus aureus by monoclonal antibodies. FEMS Microbiol. Lett. 143, 195–201.
Morfeldt, E., Tegmark, K., and Arvidson, S. 1996b. Transcriptional control of the agr-dependent virulence gene regulator, RNAIII, in Staphylococcus aureus. Mol. Microbiol. 21, 1227–1237.
Musser, J.M., Schlievert, P.M., Chow, A.W., Ewan, P., Kreiswirth, B.N., Rosdahl, V.T., Naidu, A.S., Witte, W., and Selander, R.K. 1990. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc. Natl. Acad. Sci. USA 87, 225–229.
Musthafa, K.S., Saroja, V., Pandian, S.K., and Ravi, A.V. 2011. Antipathogenic potential of marine Bacillus sp. SS4 on N-acyl-homoserine-lactone-mediated virulence factors production in Pseudomonas aeruginosa (PAO1). J. Biosci. 36, 55–67.
Nadell, C.D., Foster, K.R., and Xavier, J.B. 2010. Emergence of spatial structure in cell groups and the evolution of cooperation. PLoS Comput. Biol. 6, e1000716.
Novick, R.P., Ross, H.F., Projan, S.J., Kornblum, J., Kreiswirth, B., and Moghazeh, S. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12, 3967–3975.
Olson, M.E., Todd, D.A., Schaeffer, C.R., Paharik, A.E., Van Dyke, M.J., Büttner, H., Dunman, P.M., Rohde, H., Cech, N.B., Fey, P.D., et al. 2014. Staphylococcus epidermidis agr quorum-sensing system: Signal identification, cross talk, and importance in colonization. J. Bacteriol. 196, 3482–3493.
Otto, M. 2009. Staphylococcus epidermidis: the “accidental” pathogen. Nat. Rev. Microbiol. 7, 555–567.
Otto, M., Echner, H., Voelter, W., and Gotz, F. 2001. Pheromone cross-inhibition between Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69, 1957–1960.
Paharik, A.E., Parlet, C.P., Chung, N., Todd, D.A., Rodriguez, E.I., Van Dyke, M.J., Cech, N.B., and Horswill, A.R. 2017. Coagulasenegative staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe 22, 1–11.
Peng, H.L., Novick, R.P., Kreiswirth, B., Kornblum, J., and Schlievert, P. 1988. Cloning, characterization and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170, 4365–4372.
Rendueles, O. and Ghigo, J.M. 2012. Multi-species biofilms: how to avoid unfriendly neighbors. FEMS Microbiol. Rev. 36, 972–989.
Rendueles, O., Travier, L., Latour-Lambert, P., Fontaine, T., Magnus, J., Denamur, E., and Ghigo, J.M. 2011. Screening of Escherichia coli species biodiversity reveals new biofilm-associated antiadhesion polysaccharides. mBio 2, 1–12.
Rogers, K.L., Fey, P.D., and Rupp, M.E. 2009. Coagulase-negative staphylococcal infections. Infect. Dis. Clin. North Am. 23, 73–98.
Sakoulas, G., Eliopoulos, G.M., Moellering, R.C. Jr., Novick, R.P., Venkataraman, L., Wennersten, C., DeGirolami, P.C., Schwaber, M.J., and Gold, H.S. 2003. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J. Infect. Dis. 187, 929–938.
Sander, G., Börner, T., Kriegeskorte, A., von Eiff, C., Becker, K., and Mahabir, E. 2012. Catheter colonization and abscess formation due to Staphylococcus epidermidis with normal and small-colonyvariant phenotype is mouse strain dependent. PLoS One 7, e36602.
Schwarz, S., West, T.E., Boyer, F., Chiang, W.C., Carl, M.A., Hood, R.D., Rohmer, L., Tolker-Nielsen, T., Skerrett, S.J., and Mougous, J.D. 2010. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 6, e1001068.
Senadheera, D. and Cvitkovitch, D.G. 2008. Quorum sensing and biofilm formation by Streptococcus mutans. Adv. Exp. Med. Biol. 631, 178–188.
Shepherd, R.W. and Lindow, S.E. 2009. Two dissimilar N-acylhomoserine lactone acylases of Pseudomonas syringae influence colony and biofilm morphology. Appl. Environ. Microbiol. 75, 45–53.
Tamura, S., Yonezawa, H., Motegi, M., Nakao, R., Yoneda, S., Watanabe, H., Yamazaki, T., and Senpuku, H. 2009. Inhibiting effects of Streptococcus salivarius on dependent biofilm formation by Streptococcus mutans. Oral Microbiol. Immunol. 24, 152–161.
Tan, Y., Leonhard, M., and Schneider-Stickler, B. 2017. Evaluation of culture conditions for mixed biofilm formation with clinically isolated non-albicans Candida species and Staphylococcus epidermidis on silicone. Microb. Pathog. 112, 215–220.
Thomas, J.C., Vargas, M.R., Miragaia, M., Peacock, S.J., Archer, G.L., and Enright, M.C. 2007. Improved multilocus sequence typing scheme for Staphylococcus epidermidis. J. Clin. Microbiol. 45, 616–619.
Uçkay, I., Pittet, D., Vaudaux, P., Sax, H., Lew, D., and Waldvogel, F. 2009. Foreign body infections due to Staphylococcus epidermidis. Ann. Med. 41, 109–119.
Van Wamel, W.J.B., Van Rossum, G., Verhoef, J., Vandenbroucke-Grauls, C.M.J.E., and Fluit, A.C. 1998. Cloning and characterization of an accessory gene regulator (agr)-like locus from Staphylococcus epidermidis. FEMS Microbiol. Lett. 163, 1–9.
Wang, R., Khan, B.A., Cheung, G.Y.C., Bach, T.H., Jameson-Lee, M., Kong, K.F., Queck, S.Y., and Otto, M. 2011. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J. Clin. Invest. 121, 238–248.
Yao, Y., Sturdevant, D.E., and Otto, M. 2005. Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J. Infect. Dis. 191, 289–298.
Zhang, K., McClure, J., Elsayed, S., Louie, T., and Conly, J.M. 2005. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43, 5026–5033.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplemental material for this article may be found at http://www.springerlink.com/content/120956.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Martínez-García, S., Ortiz-García, C.I., Cruz-Aguilar, M. et al. Competition/antagonism associations of biofilm formation among Staphylococcus epidermidis Agr groups I, II, and III. J Microbiol. 57, 143–153 (2019). https://doi.org/10.1007/s12275-019-8322-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-019-8322-5