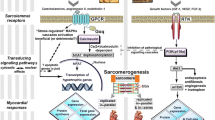
Abstract
In part 1, we considered cytomolecular mechanisms underlying calcific aortic valve disease (CAVD), hemodynamics, and adaptive feedbacks controlling pathological left ventricular hypertrophy provoked by ensuing aortic valvular stenosis (AVS). In part 2, we survey diverse signal transduction pathways that precede cellular/molecular mechanisms controlling hypertrophic gene expression by activation of specific transcription factors that induce sarcomere replication in-parallel. Such signaling pathways represent potential targets for therapeutic intervention and prevention of decompensation/failure. Hypertrophy provoking signals, in the form of dynamic stresses and ligand/effector molecules that bind to specific receptors to initiate the hypertrophy, are transcribed across the sarcolemma by several second messengers. They comprise intricate feedback mechanisms involving gene network cascades, specific signaling molecules encompassing G protein-coupled receptors and mechanotransducers, and myocardial stresses. Future multidisciplinary studies will characterize the adaptive/maladaptive nature of the AVS-induced hypertrophy, its gender- and individual patient-dependent peculiarities, and its response to surgical/medical interventions. They will herald more effective, precision medicine treatments.




Similar content being viewed by others
References
Pasipoularides, A. (2016). Calcific aortic valve disease: part 1—molecular pathogenetic aspects, hemodynamics and adaptive feedbacks. Journal of Cardiovascular Translational Research, 9, 102–118.
Krishnamurthy, V. K., Godby, R. C., Liu, G. R., et al. (2014). Review of molecular and mechanical interactions in the aortic valve and aorta: implications for the shared pathogenesis of aortic valve disease and aortopathy. Journal of Cardiovascular Translational Research, 7, 823–46.
Pasipoularides, A. (2010). Heart's vortex: intracardiac blood flow phenomena. Shelton: People's Medical Publishing House. 960 p.
Osler, W. (1892). The principles and practice of medicine: designed for the use of practitioners and students of medicine. New York: D. Appleton.
Pasipoularides, A. (2015). Mechanotransduction mechanisms for intraventricular diastolic vortex forces and myocardial deformations: part 1. Journal of Cardiovascular Translational Research, 8, 76–87.
Pasipoularides, A. (2015). Mechanotransduction mechanisms for intraventricular diastolic vortex forces and myocardial deformations: part 2. Journal of Cardiovascular Translational Research, 8, 293–318.
Barbato, E., Barton, P. J., Bartunek, J., Huber, S., Ibanez, B., Judge, D. P., Lara-Pezzi, E., Stolen, C. M., Taylor, A., & Hall, J. L. (2015). Review and updates in regenerative and personalized medicine, preclinical animal models, and clinical care in cardiovascular medicine. Journal of Cardiovascular Translational Research, 8, 466–74.
Leri, A., Kajstura, J., & Anversa, P. (2011). Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circulation Research, 109, 941–61.
Ellison, G. M., Nadal-Ginard, B., & Torella, D. (2012). Optimizing cardiac repair and regeneration through activation of the endogenous cardiac stem cell compartment. Journal of Cardiovascular Translational Research, 5, 667–77.
Marketou, M. E., Parthenakis, F., & Vardas, P. E. (2016). Pathological left ventricular hypertrophy and stem cells: current evidence and new perspectives. Stem Cells International, 2016, 5720758.
Urbanek, K., Quaini, F., Tasca, G., et al. (2003). Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America, 100, 10440–5.
Artavanis-Tsakonas, S., Rand, M. D., & Lake, R. J. (1999). Notch signaling: cell fate control and signal integration in development. Science, 284, 770–6.
Mumm, J. S., & Kopan, R. (2000). Notch signaling: from the outside in. Developmental Biology, 228, 151–65.
Bray, S. J. (2006). Notch signalling: a simple pathway becomes complex. Nature Reviews. Molecular Cell Biology, 7, 678–89.
Gude, N., & Sussman, M. (2012). Notch signaling and cardiac repair. Journal of Molecular and Cellular Cardiology, 52, 1226–32.
Elmadhun, N. Y., Sabe, A. A., Lassaletta, A. D., et al. (2014). Metabolic syndrome impairs notch signaling and promotes apoptosis in chronically ischemic myocardium. Journal of Thoracic and Cardiovascular Surgery, 148, 1048–55.
Nemir, M., & Pedrazzini, T. (2008). Functional role of Notch signaling in the developing and postnatal heart. Journal of Molecular and Cellular Cardiology, 45, 495–504.
Barry, S. P., Davidson, S. M., & Townsend, P. A. (2008). Molecular regulation of cardiac hypertrophy. International Journal of Biochemistry and Cell Biology, 40, 2023–39.
Pilegaard, H., Saltin, B., & Neufer, P. D. (2003). Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. Journal of Physiology, 546(3), 851–8.
Keeling, P.J., & Archibald, J.M. Organelle evolution: what’s in a name? Current Biology, 18, 345–347.
Blackstone, N. W. (2013). Evolution and cell physiology. 2. The evolution of cell signaling: from mitochondria to Metazoa. American Journal of Physiology. Cell Physiology, 305, C909–15.
Vega, R. B., Horton, J. L., & Kelly, D. P. (2015). Maintaining ancient organelles: mitochondrial biogenesis and maturation. Circulation Research, 116, 1820–34.
Chacinska, A., Koehler, C. M., Milenkovic, D., Lithgow, T., & Pfanner, N. (2009). Importing mitochondrial proteins: machineries and mechanisms. Cell, 138, 628–44.
Shokolenko, I., Venediktova, N., Bochkareva, A., Wilson, G. L., & Alexeyev, M. F. (2009). Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Research, 37, 2539–48.
Maulik, S. K., & Kumar, S. (2012). Oxidative stress and cardiac hypertrophy: a review. Toxicology Mechanisms and Methods, 22, 359–66.
van der Bliek, A. M., Shen, Q., & Kawajiri, S. (2013). Mechanisms of mitochondrial fission and fusion. Cold Spring Harbor Perspectives in Biology, 5, a011072.
Twig, G., Elorza, A., Molina, A. J. A., et al. (2008). Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO Journal, 27, 433–46.
Sciarretta, S., Zhai, P., Volpe, M., & Sadoshima, J. (2012). Pharmacological modulation of autophagy during cardiac stress. Journal of Cardiovascular Pharmacology, 60, 235–41.
Carafoli, E. (1797). The fateful encounter of mitochondria with calcium: how did it happen? Biochimica et Biophysica Acta, 2010, 595–606.
Winslow, R. L., Walker, M. A., & Greenstein, J. L. (2016). Modeling calcium regulation of contraction, energetics, signaling, and transcription in the cardiac myocyte. WIREs Systems Biology and Medicine, 8, 37–67.
Abel, E. D., & Doenst, T. (2011). Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovascular Research, 90, 234–42.
Droge, W. (2002). Free radicals in the physiological control of cell function. Physiological Reviews, 82, 47–95.
Lee, H. C., & Wei, Y. H. (2000). Mitochondrial role in life and death of the cell. Journal of Biomedical Science, 7, 2–15.
Levy, D., Salomon, M., D’Agostino, R. B., Belanger, A. J., & Kannel, W. B. (1994). Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation, 90, 1786–93.
Condorelli, G., Morisco, C., Stassi, G., et al. (1999). Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation, 99, 3071–8.
Spence, A. L., Naylor, L. H., Carter, H. H., et al. (2011). A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. Journal of Physiology, 589, 5443–52.
Komamura, K., Shannon, R. P., Pasipoularides, A., Ihara, T., Lader, A. S., Patrick, T. A., Bishop, S. P., & Vatner, S. F. (1992). Alterations in left ventricular diastolic function in conscious dogs with pacing-induced heart failure. Journal of Clinical Investigation, 89, 1825–38.
DeBosch, B., Treskov, I., Lupu, T. S., et al. (2006). Akt1 is required for physiological cardiac growth. Circulation, 113, 2097–104.
Dom, G. W., II, & Force, T. (2005). Protein kinase cascades in the regulation of cardiac hypertrophy. Journal of Clinical Investigation, 115, 527–37.
Molkentin, J. D. (2004). Calcineurin–NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovascular Research, 63, 467–75.
Taegtmeyer, H., Sen, S., & Vela, D. (2010). Return to the fetal gene program. Annals of the New York Academy of Sciences, 1188(1), 191–8.
Maillet, M., van Berlo, J. H., & Molkentin, J. D. (2013). Molecular basis of physiological heart growth: fundamental concepts and new players. Nature Reviews. Molecular Cell Biology, 14, 38–48.
Martin, D. E., Soulard, A., & Hall, M. N. (2004). TOR regulates ribosomal protein gene expression via PKA and the Forkhead transcription factor FHL1. Cell, 119, 969–79.
Leri, A., Claudio, P. P., Li, Q., et al. (1998). Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl- 2-to-Bax protein ratio in the cell. Journal of Clinical Investigation, 101, 1326–42.
Dweck, M. R., Boon, N. A., & Newby, D. E. (2012). Calcific aortic stenosis: a disease of the valve and the myocardium. Journal of the American College of Cardiology, 60, 1854–63.
Ricci, J. E., Munoz-Pinedo, C., Fitzgerald, P., et al. (2004). Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell, 117, 773–86.
Porrello, E. R., D’Amore, A., Curl, C. L., et al. (2009). Angiotensin II type 2 receptor antagonizes angiotensin II type 1 receptor-mediated cardiomyocyte autophagy. Hypertension, 53, 1032–40.
Dai, D. F., & Rabinovitch, P. (2011). Mitochondrial oxidative stress mediates induction of autophagy and hypertrophy in angiotensin-II treated mouse hearts. Autophagy, 7, 917–8.
Liu, S., Chen, S., Li, M., et al. (2016). Autophagy activation attenuates angiotensin II-induced cardiac fibrosis. Archives of Biochemistry and Biophysics, 590, 37–47.
Soonpaa, M. H., Kim, K. K., Pajak, L., Franklin, M., & Field, L. J. (1996). Cardiomyocyte DNA synthesis and binucleation during murine development. American Journal of Physiology. Heart and Circulatory Physiology, 271, H2183–9.
Woodcock, E. A., & Matkovich, S. J. (2005). Cardiomyocytes structure, function and associated pathologies. International Journal of Biochemistry and Cell Biology, 37, 1746–51.
Miyata, S., Minobe, W., Bristow, M. R., & Leinwand, L. A. (2000). Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circulation Research, 86, 386–90.
Backs, J., & Olson, E. N. (2006). Control of cardiac growth by histone acetylation/deacetylation. Circulation Research, 98, 15–24.
Pasipoularides, A. (2012). Diastolic filling vortex forces and cardiac adaptations: probing the epigenetic nexus. Hellenic Journal of Cardiology, 53, 458–69.
Pasipoularides, A. (2015). Linking genes to cardiovascular diseases: gene action and gene–environment interactions. Journal of Cardiovascular Translational Research, 8, 506–27.
Pasipoularides, A. (2013). Right and left ventricular diastolic pressure–volume relations: a comprehensive review. Journal of Cardiovascular Translational Research, 6, 239–52.
Pasipoularides, A., Palacios, I., Frist, W., Rosenthal, S., Newell, J. B., & Powell, W. J., Jr. (1985). Contribution of activation-inactivation dynamics to the impairment of relaxation in hypoxic cat papillary muscle. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 248, R54–62.
Pasipoularides, A., Mirsky, I., Hess, O. M., Grimm, J., & Krayenbuehl, H. P. (1986). Muscle relaxation and passive diastolic properties in man. Circulation, 74, 991–1001.
Mirsky, I., & Pasipoularides, A. (1990). Clinical assessment of diastolic function. Progress in Cardiovascular Diseases, 32, 291–318.
Mirsky, I., & Pasipoularides, A. (1980). Elastic properties of normal and hypertrophied cardiac muscle. Federation Proceeding, 39, 156–61.
Rader, F., Sachdev, E., Arsanjani, R., et al. (2015). Left ventricular hypertrophy in valvular aortic stenosis: mechanisms and clinical implications. American Journal of Medicine, 128, 344–52.
Cramariuc, D., Gerdts, E., Davidsen, E. S., Segadal, L., & Matre, K. (2010). Myocardial deformation in aortic valve stenosis: relation to left ventricular geometry. Heart, 96, 106–12.
Fielitz, J., Hein, S., Mitrovic, V., et al. (2001). Activation of the cardiac renin-angiotensin system and increased myocardial collagen expression in human aortic valve disease. Journal of the American College of Cardiology, 37, 1443–9.
Sebag, F. A., Lellouche, N., Chaachoui, N., et al. (2014). Prevalence and clinical impact of QRS duration in patients with lowflow/ low-gradient aortic stenosis due to left ventricular systolic dysfunction. European Journal of Heart Failure, 16, 639–47.
Wang, Y., & Hill, J. A. (2010). Electrophysiological remodeling in heart failure. Journal of Molecular and Cellular Cardiology, 48, 619–32.
Lipskaia, L., Chemaly, E. R., Hadri, L., Lompre, A., & Hajjar, R. J. (2010). Sarcoplasmic reticulum Ca2+ ATPase as a therapeutic target for heart failure. Expert Opinion on Biological Therapy, 10, 29–41.
Gelpi, R. J., Pasipoularides, A., Lader, A. S., et al. (1991). Changes in diastolic cardiac function in developing and stable perinephritic hypertension in conscious dogs. Circulation Research, 68, 555–67.
Vatner, S. F., Pagani, M., Manders, W. T., & Pasipoularides, A. (1980). Alpha adrenergic vasoconstriction and nitroglycerin vasodilation of large coronary arteries in the conscious dog. Journal of Clinical Investigation, 65, 5–14.
Vatner, S. F., Pasipoularides, A., & Mirsky, I. (1984). Measurement of arterial pressure-dimension relationships in conscious animals. Annals of Biomedical Engineering, 12, 521–34.
Marcus, M. L., Koyanagi, S., Harrison, D. G., Doty, D. B., Hiratzka, L. F., & Eastham, C. L. (1983). Abnormalities in the coronary circulation that occur as a consequence of cardiac hypertrophy. American Journal of Medicine, 75, 62–6.
Hittinger, L., Shannon, R. P., Bishop, S. P., Gelpi, R. J., & Vatner, S. F. (1989). Subendomyocardial exhaustion of blood flow reserve and increased fibrosis in conscious dogs with heart failure. Circulation Research, 65, 971–80.
Craig, W. E., Murgo, J. P., & Pasipoularides, A. (1987). Calculation of the time constant of relaxation. In W. Grossman & B. Lorell (Eds.), Diastolic relaxation of the heart (pp. 125–32). The Hague: Martinus Nijhoff.
Pasipoularides, A. (2015). Fluid dynamics of ventricular filling in heart failure: overlooked problems of RV/LV chamber dilatation. Hellenic Journal of Cardiology, 56, 85–95.
Galiuto, L., Lotrionte, M., Crea, F., et al. (2006). Impaired coronary and myocardial flow in severe aortic stenosis is associated with increased apoptosis: a transthoracic Doppler and myocardial contrast echocardiography study. Heart, 92, 208–12.
Ihara, T., Shannon, R. P., Komamura, K., Pasipoularides, A., Patrick, T., Shen, Y. T., & Vatner, S. F. (1994). Effects of anaesthesia and recent surgery on diastolic function. Cardiovascular Research, 28, 325–36.
Martinou, J. C. (1999). Apoptosis: key to the mitochondrial gate. Nature, 399, 411–2.
Green, D. R. (2011). Means to an end: apoptosis and other cell death mechanisms. NY: Cold Spring Harbor, Cold Spring Harbor Laboratory Press.
Weber, K. T., Sun, Y., Bhattacharya, S. K., Ahokas, R. A., & Gerling, I. C. (2012). Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nature Reviews. Cardiology, 10, 15–26.
Huusko, J., Lottonen, L., Merentie, M., et al. (2012). AAV9-mediated VEGF-B gene transfer improves systolic function in progressive left ventricular hypertrophy. Molecular Therapy, 20, 2212–21.
Olson, E. N., & Schneider, M. D. (2003). Sizing up the heart: development redux in disease. Genes and Development, 17, 1937–56.
Huss, J. M., & Kelly, D. P. (2005). Mitochondrial energy metabolism in heart failure: a question of balance. Journal of Clinical Investigation, 115, 547–55.
Paulus, W. J., Grossman, W., Serizawa, T., Bourdillon, P. D., Pasipoularides, A., & Mirsky, I. (1985). Different effects of two types of ischemia on myocardial systolic and diastolic function. American Journal of Physiology. Heart and Circulatory Physiology, 248, H719–28.
Pasipoularides, A. (1988). On mechanisms of improved ejection fraction by early reperfusion in acute myocardial infarction: myocardial salvage or infarct stiffening? [Editorial]. Journal of the American College of Cardiology, 12, 1037–8.
Spinale, F. G. (2007). Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiological Reviews, 87, 1285–342.
Weber, K. T., Janicki, J. S., Shroff, S. G., Pick, R., Chen, R. M., & Bashey, R. I. (1988). Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circulation Research, 62, 757–65.
Kassiri, Z., & Khokha, R. (2005). Myocardial extra-cellular matrix and its regulation by metalloproteinases and their inhibitors. Thrombosis and Haemostasis, 93, 212–9.
Chen, W., & Frangogiannis, N. G. (1833). Fibroblasts in post-infarction inflammation and cardiac repair. Biochimica et Biophysica Acta, 2013, 945–53.
Jugdutt, B. I. (2008). Aging and defective healing, adverse remodeling and blunted postconditioning in the wounded heart with aging. Journal of the American College of Cardiology, 51, 1399–403.
Biernacka, A., & Frangogiannis, N. G. (2011). Aging and cardiac fibrosis. Aging and Disease, 2, 158–73.
Grossman, W., Jones, D., & Mc Laurin, L. P. (1975). Wall stress and patterns of hypertrophy in the human left ventricle. Journal of Clinical Investigation, 56, 56–64.
Pasipoularides, A. (1990). Clinical assessment of ventricular ejection dynamics with and without outflow obstruction. Journal of the American College of Cardiology, 15, 859–82.
Pasipoularides, A., Murgo, J. P., Bird, J. J., & Craig, W. E. (1984). Fluid dynamics of aortic stenosis: mechanisms for the presence of subvalvular pressure gradients. American Journal of Physiology. Heart and Circulatory Physiology, 246, H542–H550.
Shim, Y., Hampton, T. G., Straley, C. A., Harrison, J. K., Spero, L. A., Bashore, T. M., & Pasipoularides, A. D. (1992). Ejection load changes in aortic stenosis: observations made following balloon aortic valvuloplasty. Circulation Research, 71, 1174–84.
Kupari, M., Laine, M., Turto, H., et al. (2013). Circulating collagen metabolites, myocardial fibrosis and heart failure in aortic valve stenosis. Journal of Heart Valve Disease, 22, 166–76.
Pasipoularides, A. D., Shu, M., Shah, A., & Glower, D. D. (2002). Right ventricular diastolic relaxation in conscious dog models of pressure overload, volume overload and ischemia. Journal of Thoracic and Cardiovascular Surgery, 124, 964–72.
Pasipoularides, A. D., Shu, M., Shah, A., Silvestry, S., & Glower, D. D. (2002). Right ventricular diastolic function in canine models of pressure overload, volume overload and ischemia. American Journal of Physiology. Heart and Circulatory Physiology, 283, H2140–H2150.
Flett, A. S., Sado, D. M., Quarta, G., et al. (2012). Diffuse myocardial fibrosis in severe aortic stenosis: an equilibrium contrast cardiovascular magnetic resonance study. European Heart Journal Cardiovascular Imaging, 13, 819–26.
Sadoshima, J., & Izumo, S. (1997). The cellular and molecular response of cardiac myocytes to mechanical stress. Annual Review of Physiology, 59, 551–71.
Hill, J. A., & Olson, E. N. (2008). Cardiac plasticity. The New England Journal of Medicine, 358, 1370–80.
Chang, H. W., Kim, K. H., Kim, J. S., et al. (2013). Relationship between morphologic features of myocardial tissue and left ventricular function in patients with aortic valve disease and left ventricular hypertrophy. Journal of Heart Valve Disease, 22, 476–83.
Levy, D., Garrison, R. J., Savage, D. D., Kannel, W. B., & Castelli, W. P. (1990). Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. The New England Journal of Medicine, 322, 1561–6.
Kupari, M., Turto, H., & Lommi, J. (2005). Left ventricular hypertrophy in aortic valve stenosis: preventive or promotive of systolic dysfunction and heart failure? European Heart Journal, 26, 1790–6.
Buermans, H. P. J., & Paulus, W. J. (2005). Iconoclasts topple adaptive myocardial hypertrophy in aortic stenosis. European Heart Journal, 26, 1697–9.
Hirt, M. N., Sörensen, N. A., Bartholdt, L. M., et al. (2012). Increased afterload induces pathological cardiac hypertrophy: a new in vitro model. Basic Research in Cardiology, 107, 1–16.
Cioffi, G., Faggiano, P., Vizzardi, E., et al. (2011). Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart, 97, 301–7.
Hosseini, M. W. (2005). Molecular tectonics: from simple tectons to complex molecular networks. Accounts of Chemical Research, 38, 313–23.
Cooper, G., Kent, R. L., Uboh, C. E., Thompson, E. W., & Marino, T. A. (1985). Hemodynamic versus adrenergic control of cat right ventricular hypertrophy. Journal of Clinical Investigation, 75, 1403–14.
Kehat, I., & Molkentin, J. D. (2010). Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation, 122, 2727–35.
Heyn, H., & Esteller, M. (2012). DNA methylation profiling in the clinic: applications and challenges. Nature Reviews. Genetics, 13, 679–92.
Kuwahara, K., Nishikimi, T., & Nakao, K. (2012). Transcriptional regulation of the fetal cardiac gene program. Journal of Pharmacological Sciences, 119, 198–203.
Ames, E. G., Lawson, M. J., Mackey, A. J., & Holmes, J. W. (2013). Sequencing of mRNA identifies re-expression of fetal splice variants in cardiac hypertrophy. Journal of Molecular and Cellular Cardiology, 62, 99–107.
Hu, C. L., Chandra, R., Ge, H., et al. (2009). Adenylyl cyclase type 5 protein expression during cardiac development and stress. American Journal of Physiology. Heart and Circulatory Physiology, 297, H1776–82.
Vatner, S. F., Park, M., Yan, L., Lee, G. J., Lai, L., Iwatsubo, K., Ishikawa, Y., Pessin, J., & Vatner, D. E. (2013). Adenylyl cyclase type 5 in cardiac disease, metabolism, and aging. American Journal of Physiology. Heart and Circulatory Physiology, 305, H1–8.
Izumo, S., Nadal-Ginard, B., & Mahdavi, V. (1988). Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proceedings of the National Academy of Sciences of the United States of America, 85, 339–43.
Pandya, K., Kim, H. S., & Smithies, O. (2006). Fibrosis, not cell size, delineates β-myosin heavy chain reexpression during cardiac hypertrophy and normal aging in vivo. Proceedings of the National Academy of Sciences of the United States of America, 103, 16864–9.
Lopez, J. E., Myagmar, B., Swigart, P. M., et al. (2011). β-Myosin heavy chain is induced by pressure overload in a minor subpopulation of smaller mouse cardiac myocytes. Circulation Research, 109, 629–38.
Pandya, K., & Smithies, O. (2011). β-MyHC and cardiac hypertrophy: size does matter. Circulation Research, 109, 609–10.
Isenberg, G., Kazanski, V., Kondratev, D., Gallitelli, M. F., Kiseleva, I., & Kamkin, A. (2003). Differential effects of stretch and compression on membrane currents and [Na+] c in ventricular myocytes. Progress in Biophysics and Molecular Biology, 82, 43–56.
Brancaccio, M., Fratta, L., Notte, A., et al. (2003). Melusin, a muscle-specific integrin β1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nature Medicine, 9, 68–75.
Sugden, P. H., & Clerk, A. (1998). ‘Stress-responsive’ mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circulation Research, 83, 345–52.
Duerr, R. L., Huang, S., Miraliakbar, H. R., Clark, R., Chien, K. R., & Ross, J., Jr. (1995). Insulin-like growth factor-1 enhances ventricular hypertrophy and function during the onset of experimental cardiac failure. Journal of Clinical Investigation, 95, 619–27.
Yamazaki, T., Komuro, I., Kudoh, S., et al. (1996). Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy. Journal of Biological Chemistry, 271, 3221–8.
Sheng, Z., Knowlton, K., Chen, J., Hoshijima, M., Brown, J. H., & Chien, K. R. (1997). Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen- activated protein kinase-dependent pathway: divergence from downstream CT-1 signals for myocardial cell hypertrophy. Journal of Biological Chemistry, 272, 5783–91.
Barnea, G., Strapps, W., Herrada, G., et al. (2008). The genetic design of signaling cascades to record receptor activation. Proceedings of the National Academy of Sciences of the United States of America, 105, 64–9.
van Berlo, J. H., Maillet, M., & Molkentin, J. D. (2013). Signaling effectors underlying pathologic growth and remodeling of the heart. Journal of Clinical Investigation, 123, 37–45.
Adams, J. W., Sakata, Y., Davis, M. G., et al. (1998). Enhanced Gαq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proceedings of the National Academy of Sciences of the United States of America, 95, 10140–5.
Sakata, Y., Hoit, B. D., Liggett, S. B., Walsh, R. A., & Dorn, G. W., II. (1998). Decompensation of pressure-overload hypertrophy in G alpha q-overexpressing mice. Circulation, 97, 1488–95.
Belmonte, S. L., & Blaxall, B. C. (2011). G protein coupled receptor kinases as therapeutic targets in cardiovascular disease. Circulation Research, 109, 309–19.
DeWire, S. M., Ahn, S., Lefkowitz, R. J., & Shenoy, S. K. (2007). Beta-arrestins and cell signaling. Annual Review of Physiology, 69, 483–510.
Waters, C., Pyne, S., & Pyne, N. J. (2004). The role of G-protein coupled receptors and associated proteins in receptor tyrosine kinase signal transduction. Seminars in Cell and Developmental Biology, 15, 309–23.
van Biesen, T., Hawes, B. E., Luttrell, D. K., et al. (1995). Receptor-tyrosine-kinase- and G beta gamma-mediated MAP kinase activation by a common signalling pathway. Nature, 376, 781–4.
Luttrell, L. M., Daaka, Y., & Lefkowitz, R. J. (1999). Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Current Opinion in Cell Biology, 11, 177–83.
Rockman, H. A., Koch, W. J., & Lefkowitz, R. J. (2002). Seven-transmembrane-spanning receptors and heart function. Nature, 415, 206–12.
Noor, N., Patel, C. B., & Rockman, H. A. (2011). Beta-arrestin: a signaling molecule and potential therapeutic target for heart failure. Journal of Molecular and Cellular Cardiology, 51, 534–41.
Port, J. D., & Bristow, M. R. (2001). Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. Journal of Molecular and Cellular Cardiology, 33, 887–905.
Packer, M., Bristow, M. R., Cohn, J. N., et al. (1996). The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. The New England Journal of Medicine, 334, 1349–55.
Cohn, J. N., & Tognoni, G. (2001). Valsartan Heart Failure Trial I. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. The New England Journal of Medicine, 345, 1667–75.
Paul, M., Poyan Mehr, A., & Kreutz, R. (2006). Physiology of local renin-angiotensin systems. Physiological Reviews, 86, 747–803.
Li, Y., Li, X. H., & Yuan, H. (2012). Angiotensin II type-2 receptor-specific effects on the cardiovascular system. Cardiovascular Diagnostic and Therapy, 2, 56–62.
Reiter, E., Ahn, S., Shukla, A. K., & Lefkowitz, R. J. (2012). Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annual Review of Pharmacology and Toxicology, 52, 179–97.
Bylund, D. B., Eikenberg, D. C., Hieble, J. P., et al. (1994). International Union of Pharmacology nomenclature of adrenoceptors. Pharmacological Reviews, 46, 121–36.
Triposkiadis, F., Karayannis, G., Giamouzis, G., et al. (2009). The sympathetic nervous system in heart failure. Journal of the American College of Cardiology, 54, 1747–62.
Feldman, D. S., Carnes, C. A., Abraham, W. T., & Bristow, M. R. (2005). Mechanisms of disease: beta-adrenergic receptors—alterations in signal transduction and pharmacogenomics in heart failure. Nature Clinical Practice. Cardiovascular Medicine, 2, 475–83.
Molkentin, J. D., & Dorn, G. W., II. (2001). Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annual Review of Physiology, 63, 391–426.
Charron, F., Paradis, P., Bronchain, O., Nemer, G., & Nemer, M. (1999). Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression. Molecular and Cellular Biology, 19, 4355–65.
Zhou, P., He, A., & Pu, W. T. (2012). Regulation of GATA4 transcriptional activity in cardiovascular development and disease. Current Topics in Developmental Biology, 100, 143–69.
Peterkin, T., Gibson, A., Loose, M., & Patient, R. (2005). The roles of GATA-4, -5 and -6 in vertebrate heart development. Seminars in Cell and Developmental Biology, 16, 83–94.
Pikkarainen, S., Tokola, H., Kerkela, R., & Ruskoaho, H. (2004). GATA transcription factors in the developing and adult heart. Cardiovascular Research, 63, 196–207.
Liang, Q., & Molkentin, J. D. (2002). Divergent signaling pathways converge on GATA4 to regulate cardiac hypertrophic gene expression. Journal of Molecular and Cellular Cardiology, 34, 611–6.
van Berlo, J. H., Elrod, J. W., van den Hoogenhof, M. M., et al. (2010). The transcription factor GATA-6 regulates pathological cardiac hypertrophy. Circulation Research, 107, 1032–40.
Akazawa, H., & Komuro, I. (2003). Roles of cardiac transcription factors in cardiac hypertrophy. Circulation Research, 92, 1079–88.
Oka, T., Maillet, M., Watt, A. J., Schwartz, R. J., Aronow, B. J., Duncan, S. A., & Molkentin, J. D. (2006). Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circulation Research, 98, 837–45.
Oka, T., Xu, J., & Molkentin, J. D. (2007). Re-employment of developmental transcription factors in adult heart disease. Seminars in Cell and Developmental Biology, 18, 117–31.
Papait, R., Kunderfranco, P., Stirparo, G. G., Latronico, M. V., & Condorelli, G. (2013). Long noncoding RNA: a new player of heart failure? Journal of Cardiovascular Translational Research, 6, 876–83.
Peters, T., & Schroen, B. (2014). Missing links in cardiology: long non-coding RNAs enter the arena. Pflügers Archiv, 466, 1177–87.
Barbato, E., Lara-Pezzi, E., Stolen, C., Taylor, A., Barton, P. J., Bartunek, J., Iaizzo, P., Judge, D. P., Kirshenbaum, L., Blaxall, B. C., Terzic, A., & Hall, J. L. (2014). Advances in induced pluripotent stem cells, genomics, biomarkers, and antiplatelet therapy highlights of the year in JCTR 2013. Journal of Cardiovascular Translational Research, 7, 518–25.
Chen, L., Qin, F., Ge, M., Shu, Q., & Xu, J. (2014). Application of adipose-derived stem cells in heart disease. Journal of Cardiovascular Translational Research, 7, 651–63.
Bernal, J. A. (2013). RNA-based tools for nuclear reprogramming and lineage-conversion: towards clinical applications. Journal of Cardiovascular Translational Research, 6, 956–68.
Inagawa, K., & Ieda, M. (2013). Direct reprogramming of mouse fibroblasts into cardiac myocytes. Journal of Cardiovascular Translational Research, 6, 37–45.
Hudson, J. E., & Porrello, E. R. (2013). The non-coding road towards cardiac regeneration. Journal of Cardiovascular Translational Research, 6, 909–23.
Wang, K., Liu, F., Zhou, L. Y., et al. (2014). The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circulation Research, 114, 1377–88.
Bartel, D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 116, 281–97.
Lim, L. P., Lau, N. C., Garrett-Engele, P., et al. (2005). Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature, 433, 769–73.
Ambros, V. (2004). The functions of animal microRNAs. Nature, 431, 350–5.
Lee, R. C., Feinbaum, R. L., & Ambros, V. (1993). The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell, 75, 843–54.
Sevignani, C., Calin, G. A., Siracusa, L. D., & Croce, C. M. (2006). Mammalian microRNAs: a small world for fine-tuning gene expression. Mammalian Genome, 17, 189–202.
van Rooij, E., Sutherland, L. B., Qi, X., Richardson, J. A., Hill, J., & Olson, E. N. (2007). Control of stress-dependent cardiac growth and gene expression by a microRNA. Science, 316, 575–9.
Catalucci, D., Gallo, P., & Condorelli, G. (2009). MicroRNAs in cardiovascular biology and heart disease. Circulation. Cardiovascular Genetics, 2, 402–8.
Latronico, M. V. G., Condorelli, G., & Dorn, G. W., II. (2010). MicroRNAs in heart disease: putative novel therapeutic targets? European Heart Journal, 31, 649–58.
Dorn, G. W., II. (2011). MicroRNAs in cardiac disease. Translational Research, 157, 226–35.
Papoutsidakis, N., Deftereos, S., Kaoukis, A., et al. (2013). MicroRNAs and the heart: small things do matter. Current Topics in Medicinal Chemistry, 13, 216–30.
Polacek, D. C., Passerini, A. G., Shi, C., et al. (2003). Fidelity and enhanced sensitivity of differential transcription profiles following linear amplification of nanogram amounts of endothelial mRNA. Physiological Genomics, 13, 147–156.
Puskás, L. G., Zvara, A., Hackler, L., Jr., & Van Hummelen, P. (2002). RNA amplification results in reproducible microarray data with slight ratio bias. BioTechniques, 32, 1330–40.
Yu, L. M., & Xu, Y. (2015). Epigenetic regulation in cardiac fibrosis. World Journal Cardiology, 7, 784–91.
Villar, A. V., Garcia, R., Merino, D., et al. (2013). Myocardial and circulating levels of microRNA-21 reflect left ventricular fibrosis in aortic stenosis patients. International Journal of Cardiology, 167, 2875–81.
Haghikia, A., & Hilfiker-Kleiner, D. (2009). MiRNA-21: a key to controlling the cardiac fibroblast compartment? Cardiovascular Research, 82, 1–3.
Cheng, Y., Ji, R., Yue, J., et al. (2007). MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? American Journal of Pathology, 170, 1831–40.
Chen, Z., Li, C., Xu, Y., et al. (2014). Circulating level of miR-378 predicts left ventricular hypertrophy in patients with aortic stenosis. PLoS One, 9(8), e105702.
Carè, A., Catalucci, D., Felicetti, F., et al. (2007). MicroRNA-133 controls cardiac hypertrophy. Nature Medicine, 13, 613–8.
Araque, J. C., Greason, K. L., Suri, R. M., et al. (2016). The role of balloon aortic valvuloplasty in patients with aortic valve stenosis and society of thoracic surgeons risk of 15% or higher. Annals of Thoracic Surgery, 101, 592–8.
Khawaja, M. Z., Sohal, M., Valli, H., et al. (2013). Standalone balloon aortic valvuloplasty: indications and outcomes from the UK in the transcatheter valve era. Catheterization and Cardiovascular Interventions, 81, 366–73.
Nishimura, R. A., Otto, C. M., Bonow, R. O., et al. (2014). AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 63, 2438–88.
Cribier, A., Eltchaninoff, H., & Tron, C. (2006). Percutaneous implantation of aortic valve prosthesis in patients with calcific aortic stenosis: technical advances, clinical results and future strategies. Journal of Interventional Cardiology, 19, S87–S96.
Clavel, M. A., Webb, J. G., Rodés-Cabau, J., et al. (2010). Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation, 122, 1928–36.
Smith, C. R., Leon, M. B., Mack, M. J., et al. (2011). Transcatheter versus surgical aortic-valve replacement in high-risk patients. The New England Journal of Medicine, 364, 2187–98.
Vizzardi, E., D'Aloia, A., Fiorina, C., et al. (2012). Early regression of left ventricular mass associated with diastolic improvement after transcatheter aortic valve implantation. Journal of the American Society of Echocardiography, 25, 1091–8.
Nagaraja, V., Raval, J., Eslick, G. D., & Ong, A. T. (2014). Transcatheter versus surgical aortic valve replacement: a systematic review and meta-analysis of randomised and non-randomised trials. Open Heart, 1, e000013.
Taniguchi, T., Morimoto, T., Shiomi, H., et al. (2015). Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. Journal of the American College of Cardiology. doi:10.1016/j.jacc.2015.10.001.
Leon, M. B., Smith, C. R., Mack, M., et al. (2010). Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. The New England Journal of Medicine, 363, 1597–607.
Beach, J. M., Mihaljevic, T., Rajeswaran, J., et al. (2014). Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. Journal of Thoracic and Cardiovascular Surgery, 147, 362–9.
Friddle, C. J., Koga, T., Rubin, E. M., & Bristow, J. (2000). Expression profiling reveals distinct sets of genes altered during induction and regression of cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America, 97, 6745–50.
Stansfield, W. E., Charles, P. C., Tang, R. H., et al. (2009). Regression of pressure-induced left ventricular hypertrophy is characterized by a distinct gene expression profile. Journal of Thoracic and Cardiovascular Surgery, 137, 232–8.
Bjornstad, J. L., Skrbic, B., Sjaastad, I., et al. (2012). A mouse model of reverse cardiac remodelling following banding-debanding of the ascending aorta. Acta Physiologica, 205, 92–102.
Pasipoularides, A., Murgo, J. P., Miller, J. W., & Craig, W. E. (1987). Nonobstructive left ventricular ejection pressure gradients in man. Circulation Research, 61, 220–7.
Sharma, U. C., Barenbrug, P., Pokharel, S., Dassen, W. R., Pinto, Y. M., & Maessen, J. G. (2004). Systematic review of the outcome of aortic valve replacement in patients with aortic stenosis. Annals of Thoracic Surgery, 78, 90–5.
Bauer, F., Coutant, V., Bernard, M., et al. (2013). Patients with severe aortic stenosis and reduced ejection fraction: earlier recovery of left ventricular systolic function after transcatheter aortic valve implantation compared with surgical valve replacement. Echocardiography, 30, 865–70.
Adams, D. H., Popma, J. J., Reardon, M. J., et al. (2014). Transcatheter aortic-valve replacement with a self-expanding prosthesis. The New England Journal of Medicine, 370, 1790–8.
Reardon, M. J., Adams, D. H., Kleiman, N. S., et al. (2015). 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. Journal of the American College of Cardiology, 66, 113–21.
Kim, S. J., Samad, Z., Bloomfield, G. S., & Douglas, P. S. (2014). A critical review of hemodynamic changes and left ventricular remodeling after surgical aortic valve replacement and percutaneous aortic valve replacement. American Heart Journal, 168, 150–9.
Hahn, R. T., Pibarot, P., Stewart, W. J., et al. (2013). Comparison of transcatheter and surgical aortic valve replacement in severe aortic stenosis: a longitudinal study of echocardiography parameters in cohort A of the PARTNER trial (placement of aortic transcatheter valves). Journal of the American College of Cardiology, 61, 2514–21.
Zajarias, A., & Cribier, A. G. (2009). Outcomes and safety of percutaneous aortic valve replacement. Journal of the American College of Cardiology, 53, 1829–36.
Milano, A. D., Faggian, G., Dodonov, M., et al. (2012). Prognostic value of myocardial fibrosis in patients with severe aortic valve stenosis. Journal of Thoracic and Cardiovascular Surgery, 144, 830–7.
Regitz-Zagrosek, V., Brokat, S., & Tschope, C. (2007). Role of gender in heart failure with normal left ventricular ejection fraction. Progress in Cardiovascular Diseases, 49, 241–51.
Devereux, R. B., Roman, M. J., Liu, J. E., et al. (2000). Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the strong heart study. American Journal of Cardiology, 86, 1090–6.
Carroll, J. D., Carroll, E. P., Feldman, T., et al. (1992). Sex associated differences in left ventricular function in aortic stenosis of the elderly. Circulation, 86, 1099–107.
Aurigemma, G. P., Silver, K. H., McLaughlin, M., Mauser, J., & Gaasch, W. H. (1994). Impact of chamber geometry and gender on left ventricular systolic function in patients > 60 years of age with aortic stenosis. American Journal of Cardiology, 74, 794–8.
Aurigemma, G. P., & Gaasch, W. H. (1995). Gender differences in older patients with pressure-overload hypertrophy of the left ventricle. Cardiology, 86, 310–7.
Douglas, P. S., Katz, S. E., Weinberg, E. O., Chen, M. H., Bishop, S. P., & Lorell, B. H. (1998). Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. Journal of the American College of Cardiology, 32, 1118–25.
Douglas, P. S., Otto, C. M., Mickel, M. C., Labovitz, A., Reid, C. L., & Davis, K. B. (1995). Gender differences in left ventricle geometry and function in patients undergoing balloon dilatation of the aortic valve for isolated aortic stenosis. NHLBI Balloon Valvuloplasty Registry. British Heart Journal, 73, 548–54.
Villari, B., Campbell, S. E., Schneider, J., Vassalli, G., Chiariello, M., & Hess, O. M. (1995). Sex-dependent differences in left ventricular function and structure in chronic pressure overload. European Heart Journal, 16, 1410–9.
Regitz-Zagrosek, V. (2006). Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nature Reviews. Drug Discovery, 5, 425–38.
Petrov, G., Dworatzek, E., Schulze, T. M., et al. (2014). Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. Journal of the American College of Cardiology: Cardiovascular Imaging, 7, 1073–80.
Dworatzek, E., Mahmoodzadeh, S., Schubert, C., et al. (2014). Sex differences in exercise-induced physiological myocardial hypertrophy are modulated by oestrogen receptor beta. Cardiovascular Research, 102, 418–28.
Kuiper, G. G., Enmark, E., Pelto-Huikko, M., Nilsson, S., & Gustafsson, J. A. (1996). Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America, 93, 5925–30.
Mosselman, S., Polman, J., & Dijkema, R. (1996). ER beta: identification and characterization of a novel human estrogen receptor. FEBS Letters, 392, 49–53.
Levy, D., Larson, M. G., Vasan, R. S., Kannel, W. B., & Ho, K. K. (1996). The progression from hypertension to congestive heart failure. JAMA, 275, 1557–62.
Petrov, G., Regitz-Zagrosek, V., Lehmkuhl, E., et al. (2010). Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation, 122, S23–S28.
Alberts, I. L., Wess, T. J., Cameron, G. J., & Laing, J. H. (2002). Structure of type I and type III heterotypic collagen fibrils: an x-ray diffraction study. Journal of Structural Biology, 137, 15–22.
Turner, N. A., & Porter, K. E. (2012). Regulation of myocardial matrix metalloproteinase expression and activity by cardiac fibroblasts. Life, 64(2), 143–50.
Fliegner, D., Schubert, C., Penkalla, A., et al. (2010). Female sex and estrogen receptor-b attenuate cardiac remodeling and apoptosis in pressure overload. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 298, 1597–1606.
Legget, M. E., Kuusisto, J., Healy, N. L., Fujioka, M., Schwaegler, R. G., & Otto, C. M. (1996). Gender differences in left ventricular function at rest and with exercise in asymptomatic aortic stenosis. American Heart Journal, 131, 94–100.
Fielitz, J., Leuschner, M., Zurbrugg, H. R., et al. (2004). Regulation of matrix metalloproteinases and their inhibitors in the left ventricular myocardium of patients with aortic stenosis. Journal of Molecular Medicine, 12, 809–20.
Mahmoodzadeh, S., Dworatzek, E., Fritschka, S., Pham, T. H., & Regitz-Zagrosek, V. (2010). 17beta-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovascular Research, 85, 719–28.
Fliegner, D., Schubert, C., Penkalla, A., et al. (2010). Female sex and estrogen receptor-β attenuate cardiac remodeling and apoptosis in pressure overload. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 298, R1597–606.
Hayashida, K., Morice, M. C., Chevalier, B., et al. (2012). Sex-related differences in clinical presentation and outcome of transcatheter aortic valve implantation for severe aortic stenosis. Journal of the American College of Cardiology, 59, 566–71.
Hamed, O., Persson, P. J., Engel, A. M., McDonough, S., & Smith, J. M. (2009). Gender differences in outcomes following aortic valve replacement surgery. International Journal of Surgery, 7, 214–7.
Stangl, V., Baldenhofer, G., Knebel, F., et al. (2012). Impact of gender on three-month outcome and left ventricular remodeling after transfemoral transcatheter aortic valve implantation. American Journal of Cardiology, 110, 884–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Fully compliant
Sources of Funding
Research support, for work from my laboratory surveyed here, was provided by National Heart, Lung, and Blood Institute, Grant R01 HL 050446; National Science Foundation, Grant CDR 8622201; and North Carolina Supercomputing Center and Cray Research.
Conflict of interest
I declare that I have no conflict of interest, whatsoever.
Ethical Approval
All procedures performed in studies involving human participants that are reviewed here were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments. All applicable international, national, and/or institutional guidelines for the care and use of animals in studies involving animals that are reviewed here were followed.
Additional information
Associate Editor Jennifer L. Hall oversaw the review of this article
Rights and permissions
About this article
Cite this article
Pasipoularides, A. Calcific Aortic Valve Disease: Part 2—Morphomechanical Abnormalities, Gene Reexpression, and Gender Effects on Ventricular Hypertrophy and Its Reversibility. J. of Cardiovasc. Trans. Res. 9, 374–399 (2016). https://doi.org/10.1007/s12265-016-9695-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-016-9695-z
Keywords
- Blood flow
- Aortic valvular stenosis
- Pressure overload
- Transvalvular gradient
- Physiological cardiac hypertrophy
- Pathological cardiac hypertrophy and failure
- Receptor tyrosine kinases
- PI3K(p110α) lipid kinase–Akt serine/threonine kinase pathway
- G protein-coupled receptors
- Mitogen-activated protein kinases
- Extracellular signal-regulated kinases 1 and 2
- Calcineurin
- Replication of cardiomyocyte sarcomeres in-parallel and in-series
- Concentric LV hypertrophy
- Subendocardial ischemia
- Fetal gene reexpression
- Cardiomyocyte apoptosis
- Myocardial fibrosis
- Mitochondrial biogenesis
- Myocardial hypertrophic and hyperplastic growth
- Resident endogenous stem/progenitor cells and myocardial regeneration