Abstract
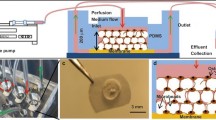
The osteocyte network is recognized as the major mechanical sensor in the bone remodeling process, and osteocyte–osteoblast communication acts as an important mediator in the coordination of bone formation and turnover. In this study, we developed a novel 3D trabecular bone explant co-culture model that allows live osteocytes situated in their native extracellular matrix environment to be interconnected with seeded osteoblasts on the bone surface. Using a low-level medium perfusion system, the viability of in situ osteocytes in bone explants was maintained for up to 4 weeks, and functional gap junction intercellular communication (GJIC) was successfully established between osteocytes and seeded primary osteoblasts. Using this novel co-culture model, the effects of dynamic deformational loading, GJIC, and prostaglandin E2 (PGE2) release on functional bone adaptation were further investigated. The results showed that dynamical deformational loading can significantly increase the PGE2 release by bone cells, bone formation, and the apparent elastic modulus of bone explants. However, the inhibition of gap junctions or the PGE2 pathway dramatically attenuated the effects of mechanical loading. This 3D trabecular bone explant co-culture model has great potential to fill in the critical gap in knowledge regarding the role of osteocytes as a mechano-sensor and how osteocytes transmit signals to regulate osteoblasts function and skeletal integrity as reflected in its mechanical properties.








Similar content being viewed by others
References
Ajubi, N. E., J. Klein-Nulend, P. J. Nijweide, T. Vrijheid-Lammers, M. J. Alblas, and E. H. Burger. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes—a cytoskeleton-dependent process. Biochem. Biophys. Res. Commun. 225:62–68, 1996.
Alford, A. I., C. R. Jacobs, and H. J. Donahue. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. Bone 33:64–70, 2003.
Bakker, A. D., M. Joldersma, J. Klein-Nulend, and E. H. Burger. Interactive effects of PTH and mechanical stress on nitric oxide and PGE2 production by primary mouse osteoblastic cells. Am. J. Physiol.-Endocrinol. Metab. 285:E608–E613, 2003.
Bakker, A. D., J. Klein-Nulend, and E. H. Burger. Mechanotransduction in bone cells proceeds via activation of COX-2, but not COX-1. Biochem. Biophys. Res. Commun. 305:677–683, 2003.
Bakker, A. D., K. Soejima, J. Klein-Nulend, and E. H. Burger. The production of nitric oxide and prostaglandin E2 by primary bone cells is shear stress dependent. J. Biomech. 34:671–677, 2001.
Bancroft, G. N., V. I. Sikavitsas, J. van den Dolder, T. L. Sheffield, C. G. Ambrose, J. A. Jansen, and A. G. Mikos. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc. Natl Acad. Sci. USA 99:12600–12605, 2002.
Brandi, M. L., and P. Collin-Osdoby. Vascular biology and the skeleton. J. Bone Miner. Res. 21:183–192, 2006.
Burger, E. H., and J. Klein-Nulend. Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J. 13:S101–S112, 1999.
Burger, E. H., J. Klein-Nulend, and J. P. Veldhuuzen. Modulation of osteogenesis in fetal bone rudiments by mechanical stress in vitro. J. Biomech. 24(Suppl. 1):101–109, 1991.
Cartmell, S. H., B. D. Porter, A. J. Garcia, and R. E. Guldberg. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 9:1197–1203, 2003.
Chambers, T. J., J. W. Chow, S. W. Fox, C. J. Jagger, and J. M. Lean. The role of prostaglandins and nitric oxide in the response of bone to mechanical stimulation. Adv. Exp. Med. Biol. 433:295–298, 1997.
Chambers, T. J., S. Fox, C. J. Jagger, J. M. Lean, and J. W. Chow. The role of prostaglandins and nitric oxide in the response of bone to mechanical forces. Osteoarthritis Cartilage 7:422–423, 1999.
Cherian, P. P., A. J. Siller-Jackson, S. Gu, X. Wang, L. F. Bonewald, E. Sprague, and J. X. Jiang. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol. Biol. Cell 16:3100–3106, 2005.
Chow, J. W. Role of nitric oxide and prostaglandins in the bone formation response to mechanical loading. Exerc. Sport Sci. Rev. 28:185–188, 2000.
Dallas, S. L., G. Zaman, M. J. Pead, and L. E. Lanyon. Early strain-related changes in cultured embryonic chick tibiotarsi parallel those associated with adaptive modeling in vivo. J. Bone Miner. Res. 8:251–259, 1993.
David, V., A. Guignandon, A. Martin, L. Malaval, M. H. Lafage-Proust, A. Rattner, V. Mann, B. Noble, D. B. Jones, and L. Vico. Ex vivo bone formation in bovine trabecular bone cultured in a dynamic 3D bioreactor is enhanced by compressive mechanical strain. Tissue Eng. Part A 14:117–126, 2008.
Davies, C. M., D. B. Jones, M. J. Stoddart, K. Koller, E. Smith, C. W. Archer, and R. G. Richards. Mechanically loaded ex vivo bone culture system ‘Zetos’: systems and culture preparation. Eur. Cell Mater. 11:57–75, 2006; discussion 75.
El-Haj, A. J., S. L. Minter, S. C. Rawlinson, R. Suswillo, and L. E. Lanyon. Cellular responses to mechanical loading in vitro. J. Bone Miner. Res. 5:923–932, 1990.
Genetos, D. C., C. J. Kephart, Y. Zhang, C. E. Yellowley, and H. J. Donahue. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J. Cell. Physiol. 212:207–214, 2007.
Hoffler, C. E., K. D. Hankenson, J. D. Miller, S. K. Bilkhu, and S. A. Goldstein. Novel explant model to study mechanotransduction and cell-cell communication. J. Orthop. Res. 24:1687–1698, 2006.
Hung, C. T., F. D. Allen, S. R. Pollack, and C. T. Brighton. Intracellular Ca2+ stores and extracellular Ca2+ are required in the [Ca2+]i response of bone cells experiencing fluid flow. J. Biomech. 29(11):1403–1409, 1996.
Jacobs, C. R., C. E. Yellowley, B. R. Davis, Z. Zhou, J. M. Cimbala, and H. J. Donahue. Differential effect of steady versus oscillating flow on bone cells. J. Biomech. 31:969–976, 1998.
Jiang, J. X., A. J. Siller-Jackson, and S. Burra. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front. Biosci. 12:1450–1462, 2007.
Malone, A. M., C. T. Anderson, P. Tummala, R. Y. Kwon, T. R. Johnston, T. Stearns, and C. R. Jacobs. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc. Natl Acad. Sci. USA 104:13325–13330, 2007.
Mann, V., C. Huber, G. Kogianni, D. Jones, and B. Noble. The influence of mechanical stimulation on osteocyte apoptosis and bone viability in human trabecular bone. J. Musculoskelet. Neuronal Interact. 6:408–417, 2006.
Nauman, E. A., R. L. Satcher, T. M. Keaveny, B. P. Halloran, and D. D. Bikle. Osteoblasts respond to pulsatile fluid flow with short-term increases in PGE2 but no change in mineralization. J. Appl. Physiol. 90:1849–1854, 2001.
Norvell, S. M., S. M. Ponik, D. K. Bowen, R. Gerard, and F. M. Pavalko. Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3-E1 osteoblasts does not require intact microfilaments or microtubules. J. Appl. Physiol. 96:957–966, 2004.
Pitsillides, A. A., S. C. Rawlinson, R. F. Suswillo, S. Bourrin, G. Zaman, and L. E. Lanyon. Mechanical strain-induced NO production by bone cells: a possible role in adaptive bone (re)modeling? FASEB J. 9:1614–1622, 1995.
Rawlinson, S. C., A. J. El-Haj, S. L. Minter, I. A. Tavares, A. Bennett, and L. E. Lanyon. Loading-related increases in prostaglandin production in cores of adult canine cancellous bone in vitro: a role for prostacyclin in adaptive bone remodeling? J. Bone Miner. Res. 6:1345–1351, 1991.
Rawlinson, S. C., C. P. Wheeler-Jones, and L. E. Lanyon. Arachidonic acid for loading induced prostacyclin and prostaglandin E2 release from osteoblasts and osteocytes is derived from the activities of different forms of phospholipase A2. Bone 27:241–247, 2000.
Siller-Jackson, A. J., S. Burra, S. Gu, X. Xia, L. F. Bonewald, E. Sprague, and J. X. Jiang. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J. Biol. Chem. 283:26374–26382, 2008.
Takai, E., R. L. Mauck, C. T. Hung, and X. E. Guo. Osteocyte viability and regulation of osteoblast function in a 3D trabecular bone explant under dynamic hydrostatic pressure. J. Bone Miner. Res. 19:1403–1410, 2004.
Takeda, S., and G. Karsenty. Molecular bases of the sympathetic regulation of bone mass. Bone 42:837–840, 2008.
Tan, S. D., A. M. Kuijpers-Jagtman, C. M. Semeins, A. L. Bronckers, J. C. Maltha, J. W. Von den Hoff, V. Everts, and J. Klein-Nulend. Fluid shear stress inhibits TNFalpha-induced osteocyte apoptosis. J. Dent. Res. 85:905–909, 2006.
Tatsumi, S., K. Ishii, N. Amizuka, M. Li, T. Kobayashi, K. Kohno, M. Ito, S. Takeshita, and K. Ikeda. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 5:464–475, 2007.
Taylor, A. F., M. M. Saunders, D. L. Shingle, J. M. Cimbala, Z. Zhou, and H. J. Donahue. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am. J. Physiol. Cell Physiol. 292:C545–C552, 2007.
Vance, J., S. Galley, D. F. Liu, and S. W. Donahue. Mechanical stimulation of MC3T3 osteoblastic cells in a bone tissue-engineering bioreactor enhances prostaglandin E2 release. Tissue Eng. 11:1832–1839, 2005.
Watanuki, M., A. Sakai, T. Sakata, H. Tsurukami, M. Miwa, Y. Uchida, K. Watanabe, K. Ikeda, and T. Nakamura. Role of inducible nitric oxide synthase in skeletal adaptation to acute increases in mechanical loading. J. Bone Miner. Res. 17:1015–1025, 2002.
Yellowley, C. E., Z. Li, Z. Zhou, C. R. Jacobs, and H. J. Donahue. Functional gap junctions between osteocytic and osteoblastic cells. J. Bone Miner. Res. 15:209–217, 2000.
Zaman, G., S. L. Dallas, and L. E. Lanyon. Cultured embryonic bone shafts show osteogenic responses to mechanical loading. Calcif. Tissue Int. 51:132–136, 1992.
Acknowledgments
We would like to thank Ms. Jiasi Chen for her assistance in histology. This work was supported by NIH grant R21 AR052417 (X. Edward Guo).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, M.E., Lu, X.L., Huo, B. et al. A Trabecular Bone Explant Model of Osteocyte–Osteoblast Co-Culture for Bone Mechanobiology. Cel. Mol. Bioeng. 2, 405–415 (2009). https://doi.org/10.1007/s12195-009-0075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-009-0075-5