Abstract
Objective
The objective was to define the toxicity and activity of weekly docetaxel administered with a short course of estramustine and enoxaparine in patients with hormone-resistant prostate cancer (HRPC).
Patients and methods
Twenty-four patients were treated with the next regimen: weekly docetaxel 36 mg/m2 iv for three consecutive weeks every 28 days, and estramustine 280 mg three times a day for three consecutive days beginning the day before docetaxel (days 1–3, 8–10 and 15–17). In order to prevent thromboembolic events, 40 mg of subcutaneous enoxaparine was administered daily sc on the same days as estramustine. Primary endpoints were: toxicity, especially the presence of thromboembolic events, PSA response rate and response in measurable disease. Secondary endpoints were: time to PSA progression and overall survival.
Results
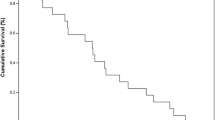
Nineteen of 24 patients (79.1%, 95% CI 71–87%) had a PSA response ≥50%. Four of the eleven patients with measurable disease had a partial response. The median time to PSA progression was 7 months (CI 95%: 6.5–9) and the median survival was 19 months (IC 95%: 11–24). Toxicity was manageable with no treatment-related mortality. Only two patients had grade 4 neutropenia. Two patients had thrombotic events, one deep venous thrombosis and one stroke. The main grade 3 non-haematologic toxicity was diarrhoea and asthenia, both in 25% of patients.
Conclusions
Weekly docetaxel with a short course of estramustine and enoxaparine is active and tolerable in HRPC patients. The observed incidence of thrombosis was lower than previously reported but the association of enoxaparine was not enough to completely prevent the thromboembolic events.
Similar content being viewed by others
References
Jemal A, Siegel R, Ward E et al (2007) Cancer Statistics 2007. CA Cancer J Clin 57:43–66
Small E, Halabi S, Dawson MA et al (2004) Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol 22:1025–1033
Tannock IF, Osoba D, Stokler MR et al (1996) Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer. A Canadian randomized trial with palliative end points. J Clin Oncol 14:1756–1764
Kantoff PW, Halabi S, Conaway M et al (1999) Hydrocortisone with or without mitoxantrone in men with hormone refractory prostate cancer. Results of the Cancer and Leukemia Group B9182 Study. J Clin Oncol 17:2506–2513
Mcdonnell TJ, Navone NM, Troncoso P et al (1997) Expression of Bcl-2 oncoprotein and p53 protein accumulation in bone marrow metastases of androgen independent prostate cancer. J Urol 157:569–574
Stein CA (1999) Mechanisms of action of taxanes in prostate cancer. Semin Oncol 26[Suppl 17]:3–7
Dahllof B, Billstrom A, Cabral F et al (1993) Estramustine depolymerizes microtubules by binding to tubulin. Cancer Res 53:4573–4581
Kreis W, Budman DR, Calabro A et al (1997) Unique synergism or antagonism of combinations of chemotherapeutic and hormonal agents in human prostate cancer cell lines. Br J Urol 79:196–202
Petrylak DP, Macarthur RB, O’Connor J et al (1999) Phase I trial of docetaxel with estramustine in androgen-independent prostate cancer. J Clin Oncol 17:958–967
Kreis W, Budman DR, Fetten J et al (1999) Phase I trial of the combination of daily estramustine phosphate and intermittent docetaxel in patients with metastatic hormone refractory prostate carcinoma. Ann Oncol 10:33–38
Savarese D, Halabi S, Hars V et al (2001) A phase II study of docetaxel, estramustine, and low dose hydrocortisone in men with hormone refractory prostate cancer: a final report of CALGB 9780. J Clin Oncol 19:2509–2516
Sinibaldi VJ, Carducci MA, Moore-Cooper S et al (2002) Phase II evaluation of docetaxel plus one-day oral estramustine phosphate in the treatment of patients with androgen independent prostate carcinoma. Cancer 94:1457–1465
Petrylak DP, Shelton GB, England-Owen C et al (2000) Response and preliminary survival results of a phase II study of docetaxel + estramustine in patients with androgen-independent prostate cancer (abstract). Proc Am Soc Clin Oncol 19:334a
Copur MS, Tarantolo SR, Hauke R et al (2000) Weekly estramustine, taxotere and dexamethasone in patients with hormone refractory prostate cancer (abstract). Proc Am Soc Clin Oncol 19:347a
Oudard S, Banu E, Beuzeboc P et al (2005) Multicenter randomized phase II study of two schedules of docetaxel, estramustine, and prednisone versus mitoxantrone plus prednisone in patients with metastatic hormone-refractory prostate cancer. J Clin Oncol 20:3343–3351
Petrylak DP, Tangen CM, Hussain MH et al (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513–1520
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Halabi S, Small EJ, Kantoff PW et al (2003) Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol 21:1232
Lubiniecki GM, Berlin JA, Weinstein RB et al (2004) Thromboembolic events with estramustine phosphate-based chemotherapy in patients with hormone-refractory prostate carcinoma: results of a meta-analysis. Cancer 101:2755–2759
Nelius T, Klatte T, Yap R et al (2006) A randomized study of docetaxel and dexamethasone with low-or high-dose estramustine for patients with advanced hormone-refractory prostate cancer. BJU Int 98:580–585
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Martín, A., Fernández, E., Vaz, M.A. et al. Long-term outcome of a phase II study of weekly docetaxel with a short course of estramustine and enoxaparine in hormone-resistant prostate cancer patients. Clin Transl Oncol 9, 323–328 (2007). https://doi.org/10.1007/s12094-007-0060-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-007-0060-1