Abstract
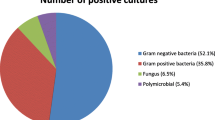
Empirical antimicrobial therapy is usually started in febrile neutropenic patients without having culture results. The aim of this study was to help determine the policies of empirical antibiotic usage in febrile neutropenic children by detecting the antimicrobial susceptibility profile in this group of patients. In this study 811 blood cultures taken from neutropenic children hospitalized at the Department of Oncology of Gaziantep Children Hospital November 2007 and February 2010 were retrospectively evaluated. Blood cultures were routinely collected in aerobic and anaerobic media and incubated using the BACTEC system. Identification and antimicrobial susceptibility testing of the isolates to antimicrobial agents was performed using the Vitek2® system according to the recommendations of the Clinical and Laboratory Standards Institute. Of 811 isolates analyzed, 128 (56.4%) were gram positive cocci, 43 (18.9%) were gram negative bacilli and fungi accounted for 56 (24.7%). The main isolated Gram-positive bacteria from blood were coagulase-negative staphylococcus (56.7%), followed by methicillin-resistant Staphylococcus aureus (14.1%). S. aureus and Streptococcus spp. were all susceptible to linezolid, vancomycin and teicoplanin. S aureus was still susceptible to few other antimicrobial agents such as tetracycline (82.4%), chloramphenicol (55.6%). Seven E. faecium, 7 E. fecalis and 1 E. hirae was isolated from blood cultures. Vancomycin resistance was detected in 6 out of 15 (40%) Enterococcus spp. isolates. Among gram-negative bacteria E. coli (30.2%) was followed by Klebsiella pneumoniae (20.9%) and Proteus spp. (18.6%). Imipenem (89.2%), meropenem (86.6%), chloramphenicol (88.9%), amicasin (82.4%) and fosfomycin (81.3%) showed highest susceptibility in vitro activity against all Gram-negative isolates. To know the antimicrobial susceptibility profile of the pathogens frequently isolated from febrile neutropenic children and to consider this profile before starting an empirical antibiotic therapy would help the clinics which have any role in the treatment of these patients to determine the empirical antibiotic usage policies.
Similar content being viewed by others
References
Klastersky J (1998) Science and pragmatism in the treatment and prevention of neutropenic infection. J Antimicrob Chemother 41:13–24
Akova M (2006) Emerging problem pathogens: a review of resistance patterns over time. Int J Infect Dis 10:S3–S8
Clinical and Laboratory Standards Institute (2009) Performance standards for antimicrobial susceptibility testing. Nineteenth informational supplement. M100-S19. CLSI, Wayne, PA
Zinner SH (1999) Changing epidemiology of infections in patients with neutropenia and cancer: emphasis on gram-positive and resistant bacteria. Clin Infect Dis 29:490–494
Ramphal R (2004) Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated agents. Clin Infect Dis 39(suppl 1):S25–S31
De Lalla F (1997) Antibiotic treatment of febrile episodes in neutropenic cancer patients. Drugs 53:789–804
Elting L, Rubenstein E, Rolston K, Bodey G (1997) Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clin Infect Dis 25:247–259
Ariffin H, Navaratnam P, Lin HP (2002) Surveillance study of bacteremic episodes in febrile neutropenic children. Int J Clin Pract 56:237–240
Paya E, Alvarez AM, Aviles C et al (2001) Causative agents of bloodstream infections in children with neoplasm, in 5 hospitals of Santiago. Rev Med Chil 129:1297–1304
Viscoli C, Castagnola E, Giacchino M et al (1999) Bloodstream infections in children with cancer: a multicentre surveillance study of the Italian Association of Pediatric Haematology and Oncology. Supportive Therapy Group-Infectious Diseases Section. Eur J Cancer 35:770–774
Calik N et al (2005) Epidemiology of bacterial infections and risk factors for mortality in cancer patients. In: Abstracts ICAAC, Washington DC, USA. K-1529
Meropenem Study Group of Turkey, Akova M, Akan H (1999) Comparison of meropenem with amikacin plus ceftazidime in the empirical treatment of febrile neutropenia: a prospective randomised multicentre trial in patients without previous prophylactic antibiotics. Int J Antimicrob Agents 13:15–19
Denning DW et al (1997) Guidelines fort he investigation of invasive fungal infections in haematological malignancy and solid organ transplantation. Eur J Clin Microbiol Infect Dis 16:424–436
Oppenheim BA (1998) The changing pattern of infection in neutropenic patients. J Antimicrob Chemother 41(D):7–11
Viscoli C, Castagnola E (2002) Treatment of febrile neutropenia: what is new? Curr Opin Infect Dis 15:377–382
Edmond MB, Ober JF, Weinbaum DL et al (1995) Vancomycin-resistant Enterococcus faecium bacteremia: risk factors for infection. Clin Infect Dis 20:1126–1133
Mlynarczyk A, Mlynarczyk G, Luczak M (2002) Conjugative transfer of glycopeptide and macrolide resistant genes among enterococci and from Enterococcus faecalis to Staphylococcus aureus. Med Dosw Mikrobiol 54:21–28
Noble WC, Virani Z, Cree RG (1992) Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett 72:195–198
Carratalá J, Alcaide F, Fernández Sevilla A et al (1995) Bacteremia due to viridans streptococci that are highly resistant to penicillin: increase among neutropenic patients with cancer. Clin Infect Dis 20:1169–1173
Borg MA, Scicluna E, de Kraker M, van de Sande-Bruinsma N, Tiemersma E, Gur D, Ben Redjeb S, Rasslan O, Elnassar Z, Benbachir M, Pieridou Bagatzouni D, Rahal K, Daoud Z, Grundmann H, Monen J (2006) Antibiotic resistance in the southeastern Mediterranean—preliminary results from the ARMed project. Euro Surveill 11:164–167
Celkan T, Diren S, Ozyilmaz I, Karaman S, Canbolat A, Ozkan A, Apak H, Yildiz I (2006) The growth rates, isolated agents and their antibiotic resistance in febrile neutropenic attacks between 2000–2004 years. ANKEM Dergisi 20:4–9
Kosack A, Riedel E, Kiehn TE, Small TN, Wexler LH, Dunkel IJ (2009) Vancomycin-resistant enterococcus in pediatric oncology patients: an analysis of potential consequences of colonization and infection. Pediatr Blood Cancer 52:300–302
Werner G, Coque TM, Hammerum AM, Hope R, Hryniewicz W, Johnson A, Klare I, Kristinsson KG, Leclercq R, Lester CH, Lillie M, Novais C, Olsson-Liljequist B, Peixe LV, Sadowy E, Simonsen GS, Top J, Vuopio-Varkila J, Willems RJ, Witte W, Woodford N (2008) Emergence and spread of vancomycin resistance among enterococci in Europe. Euro Surveill 13pii:19046
Schouten MA, Hoogkamp-Korstanje JA, Meis JF et al (2000) Prevalence of vancomycin-resistant enterococci in Europe. Eur J Clin Microbiol Infect Dis 19:816–822
Kilic A, Bedir O, Tunc T, Besirbellioglu B, Eyigun CP, Basustaoglu AC (2009) An outbreak of vanA genotype Enterococcus faecium in pediatric clinic of a training hospital. Mikrobiyol Bul 43:365–372
Ergani-Ozcan A, Naas T, Baysan BO, Ogunc D, Inan D, Colak D, Nordmann P (2008) Nosocomial outbreak of vancomycin-resistant Enterococcus faecium in a paediatric unit at a Turkish university hospital. J Antimicrob Chemother 61:1033–1039
Comert FB, Kulah C, Aktas E, Ozlu N, Celebi G (2007) First isolation of vancomycin-resistant enteroccoci and spread of a single clone in a university hospital in northwestern Turkey. Eur J Clin Microbiol Infect Dis 26:57–61
Kacmaz B, Aksoy A (2005) Antimicrobial resistance of enterococci in Turkey. Int J Antimicrob Agents 25:535–538
Rolston KV, Berkey P, Bodey GP et al (1992) A comparison of imipenem to ceftazidime with or without amikacin as empiric therapy in febrile neutropenic patients. Arch Intern Med 152:283–291
Yamamura D, Gucalp R, Carlisle P et al (1997) Open randomized study of cefepime versus piperacillin-gentamicin for treatment of febrile neutropenic cancer patients. Antimicrob Agents Chemother 41:1704–1708
EORTC, Dornbusch K, King A, Legakis N (1998) Incidence of antibiotic resistance in blood and urine isolates from hospitalized patients. Report from a European collaborative study. European Study Group on Antibiotic Resistance (ESGAR). Scand J Infect Dis 30:281–288
Verbist L (1993) Epidemiology and sensitivity of 8625 ICU and haematology/oncology bacterial isolates in Europe. International Study Group. Scand J Infect Dis Suppl 91:14–24
2003. NN-V. Usage of Antibacterial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø/Oslo 2004, 2004
Butt T, Afzal RK, Ahmad RN et al (2004) Bloodstream infections in febril neutropenic patients: bacterial spectrum and antimicrobial susceptibility pattern. J Ayub Med Coll Abbottabad 16:18–22
Ariffin H et al (2000) Ceftazidime resistant Klebsiella pneumoniae bloodstream infection in children with febrile neutropenia. Int J Infect Dis 4:21–25
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aslan, S., Citak, E.C., Yis, R. et al. Bacterial Spectrum and Antimicrobial Susceptibility Pattern of Bloodstream Infections in Children with Febrile Neutropenia: Experience of Single Center in Southeast of Turkey. Indian J Microbiol 52, 203–208 (2012). https://doi.org/10.1007/s12088-011-0210-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-011-0210-6