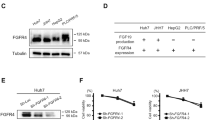
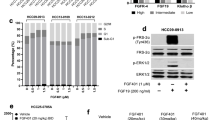
Abstract
Background
Hepatocellular carcinoma (HCC) is the most common liver cancer globally, claiming nearly 1 million lives each year. The overexpression of fibroblast growth factor (FGF) receptors (FGFRs) signaling cascade has been shown to contribute to tumorigenesis, metastasis, and poor prognosis in HCC. Therefore, targeted inhibition of the FGF/FGFR cascade may represent a new treatment strategy for HCC patients.
Methods
HCC patient-derived xenograft (PDX) models were implanted into either severe combined immunodeficient (SCID) or CD34+hu-NSG (humanized) mice and subsequently treated with vehicle, infigratinib (FGFR1-3 inhibitor), FGF401 (FGFR4 inhibitor), or the combination of infigratinib and FGF401. Tumor progressions, overall survival of mice, lung metastasis, and drug resistance were monitored, and samples collected at the end of the treatment cycle were subjected to Western blot analyses and immunohistochemistry.
Results
HCC PDX models expressing high levels of FGF19/FGFR4 or FGFR2/3 showed favorable initial treatment response to FGF401 and infigratinib, respectively. However, progressive disease due to acquired resistance was observed. Combination infigratinib/FGF401 augmented the antitumor activity, response rate, and overall survival of mice. This combination significantly increased the infiltration of B cells, macrophages, CD8+ T cells, and CD4+ T cells associated with granzyme-B-mediated apoptosis, delayed onset of resistance, and inhibited metastasis by potently inhibiting several critical signaling pathways involved in proliferation and metastasis.
Conclusions
Our findings suggest that HCC patients with high FGFR2/3 or FGF19/FGFR4 expressing tumors might benefit from a combination infigratinib/FGF401; thus, supporting its evaluation in clinical trials.






Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- bFGF:
-
Basic fibroblast growth factor
- CYR61:
-
Cysteine-rich angiogenic inducer 61
- FGF:
-
Fibroblast growth factor
- FGFR:
-
Fibroblast growth factor receptor
- HCC:
-
Hepatocellular carcinoma
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- OS:
-
Overall survival
- PDGF-AA:
-
Platelet-derived growth factor with two A subunits
- PDX:
-
Patient derived xenograft
- SCID:
-
Severe combined immunodeficient
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
References
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30
Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol 2020;38:4317–4345
Harimoto N, Taguchi K, Shirabe K, Adachi E, Sakaguchi Y, Toh Y, et al. The significance of fibroblast growth factor receptor 2 expression in differentiation of hepatocellular carcinoma. Oncology 2010;78:361–368
Paur J, Nika L, Maier C, Moscu-Gregor A, Kostka J, Huber D, et al. Fibroblast growth factor receptor 3 isoforms: novel therapeutic targets for hepatocellular carcinoma? Hepatology 2015;62:1767–1778
Kano MR, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y, et al. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci 2005;118:3759–3768
Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008;8:592–603
Hyeon J, Ahn S, Lee JJ, Song DH, Park C-K. Expression of fibroblast growth factor 19 is associated with recurrence and poor prognosis of hepatocellular carcinoma. Dig Dis Sci 2013;58:1916–1922
Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu J-Y, et al. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res 2014;74:3306–3316
French DM, Lin BC, Wang M, Adams C, Shek T, Hotzel K, et al. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS One 2012;7:e36713
Sawey ET, Chanrion M, Cai C, Wu G, Zhang J, Zender L, et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell 2011;19:347–358
Guagnano V, Furet P, Spanka C, Bordas V, Le Douget M, Stamm C, et al. Discovery of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamino]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J Med Chem 2011;54:7066–7083
Huynh H, Lee LY, Goh KY, Ong R, Hao H-X, Huang A, et al. Infigratinib mediates vascular normalization, impairs metastasis, and improves chemotherapy in hepatocellular carcinoma. Hepatology 2019;69:943–958
Weiss A, Adler F, Buhles A, Stamm C, Fairhurst RA, Kiffe M, et al. FGF401, a first-in-class highly selective and potent FGFR4 inhibitor for the treatment of FGF19-driven hepatocellular cancer. Mol Cancer Ther 2019;18:2194–2206
Huynh H, Prawira A, Le TBU, Vu TC, Hao H-X, Huang A, et al. FGF401 and vinorelbine synergistically mediate antitumor activity and vascular normalization in FGF19-dependent hepatocellular carcinoma. Exp Mol Med 2020;52:1857–1868
Chan SL, Yen C-J, Schuler M, Lin C-C, Choo SP, Weiss K-H, et al. Abstract CT106: Ph I/II study of FGF401 in adult pts with HCC or solid tumors characterized by FGFR4/KLB expression. Cancer Res 2017;77:CT106LP
Guide for the Care and Use of Laboratory Animals, 8th edition. Washington (DC): The National Academies Press; 2011.
Huynh H, Ngo VC, Koong HN, Poon D, Choo SP, Toh HC, et al. AZD6244 enhances the anti-tumor activity of sorafenib in ectopic and orthotopic models of human hepatocellular carcinoma (HCC). J Hepatol 2010;52:79–87
Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005;8:299–309
Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, et al. Synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in murine hepatocellular carcinoma. Hepatology 2002;35:834–842
Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734–1736
Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 2017;544:250–254
Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 2018;15:310–324
Kurebayashi Y, Ojima H, Tsujikawa H, Kubota N, Maehara J, Abe Y, et al. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 2018;68:1025–1041
Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–567
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–571
Chen P-L, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 2016;6:827–837
Nogova L, Sequist LV, Perez Garcia JM, Andre F, Delord J-P, Hidalgo M, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1–3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion St. J Clin Oncol 2017;35:157–165
Dawkins J, Webster RM. The hepatocellular carcinoma market. Nat Rev Drug Discov 2019;18:13–14
Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther 2001;298:865–872
Funding
This work was supported by Singapore National Medical Research Council–Ministry of Health Industry Alignment Fund Category 2 Grant NMRC/MOHIAFCAT2/006/2016 (H.H.), Singapore National Medical Research Council–Ministry of Health Industry Alignment Fund Category 1 Grant NMRC/MOHIAFCat1/0026/2015 and NMRC/MOHIAFCat1/0009/2014 (H.H.), the RIE2020 NCIS Centre Grant, grant number CGAug16M005 (H. H.), and Singapore National Research Foundation NRF-CRP17-2017–05 (H.H.).
Author information
Authors and Affiliations
Contributions
Conception and study design: HH and DTWM. Development of methodology: HH. Acquisition of data: TBUL, AP, RHZW. Analysis and interpretation of data: DTWM and HH. Writing, review, and/or revision of manuscript: DTWM, TBUL, AP, and HH. Study supervision: HH.
Corresponding author
Ethics declarations
Conflict of interest
David Tai Wai Meng, Thi Bich Uyen Le, Aldo Prawira, Rebecca Ho Zhi Wen, Hung Huynh declare that they have no conlict of interest.
Ethical approval
This study received ethics board approval at SingHealth and National Cancer Centre Singapore (IACUC #2018/SHS/1401 and #2012/SHS/731). This study involves the use of human biological material (patient-derived xenografts) that is not individually identifiable.
Consent to participate
Consent to collect tumor samples from patients were obtained by the SingHealth Tissue Repository prior to tissue manipulation to generate xenografts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12072_2021_10212_MOESM2_ESM.pdf
Supplementary file2 Supplementary Data 1. Effects of infigratinib, FGF401, and infigratinib/FGF401 on tumor growth of HCC models. High FGFR2/3-expressing HCC01-0909 (A), high FGFR2/3-expressing HCC06-0606 (B), high FGF19-expressing HCC26-1004 (C), high FGF19-expressing HCC13-0212 (D), low FGFR-expressing HCC10-0505 (E), and high FGFR2/3-expressing sorafenib-resistant HCC06-0606Sor87 (F) were implanted subcutaneously into SCID mice as described in Materials and Methods. Mice bearing indicated tumors were randomized and divided into four groups. They treated as described in Figure 1 for the indicated days. Lenvatinib was given at the dose of 10 mg/kg/day. Each treatment arm comprised 8–10 independent tumor-bearing mice. Tumor growth was monitored and tumor volumes over time were calculated as previously described [12] and are shown. Supplementary Data 2. Toxicity associated with infigratinib/FGF401 treatment. High FGFR2/3-expressing HCC01-0909 tumors were implanted subcutaneously into SCID mice as described in Materials and Methods. Mice bearing HCC01-0909 tumors were orally treated with 1) vehicle (control), 2) 15 mg/kg infigratinib once daily, 3) 30 mg/kg FGF401 twice daily, or 4) indicated combined infigratinib and FGF401 as described in Figure 2. Additional four groups were treated with vehicle and four combinations in a step-down fashion (15:30, 10:20, 7.5:15, and 5:10 infigratinib:FGF401) for 15 days. Each group consisted of eight mice. Treatment was initiated when the tumors reached sizes of approximately 170–250 mm3. Representative photographs of mice from vehicle- and drug-treated mice were taken at the end of the treatments and are shown (A). Throughout the course of treatment, no significant body weight loss or other clinical signs of toxicity were observed in the mice of the treatment groups compared to those of the vehicle group, indicating that the toxicity associated with infigratinib/FGF401 is acceptable. (B) Full blood count and serum derived from mice treated with vehicle, infigratinib, FGF401, and infigratinib plus FGF401 as described in Figure 1 for 21 days were analyzed using the Preventive Care Profile Plus (Abaxis, Inc, Union City, CA, USA) according to the manufacturer’s instructions. Treatment of mice with infigratinib resulted in an elevation in serum alkaline phosphatase (ALP), alanine transaminase (ALT), and aspartate transaminase (AST), indicative of liver damage. These observations were consistent with the safety profiles of infigratinib in human studies [27, 28]. The addition of FGF401 to infigratinib did not cause further elevation of serum ALT, ALP, and AST compared to infigratinib or FGF401monotherapy. Supplementary Data 3. Effects of infigratinib, FGF401, and infigratinib/FGF401 on cell cycle of HCC01-0909 models. HCC01-0909 cells were plated at a density of 5 x 105 and then treated with either 0.1% DMSO, 0.5 µM of infigratinib, 1.0 µM of FGF401, or the indicated doses of infigratinib/FGF401 in growth medium for 24h. Cell cycle analysis was performed as described in Supplementary Materials and Methods and cell cycle distribution is shown. Differences in cell cycle distribution were compared using Student’s t test. *p < 0.05; **p < 0.01; ***p < 0.001. Supplementary Data 4. Effects of infigratinib/FGF401 on angiogenesis, cell proliferation, and apoptosis. Mice bearing high FGF19 expressing-HCC13-0212, and low FGFR2/3-expressing HCC10-0505 tumors were treated with infigratinib, FGF401, and infigratinib/FGF401 for 10 days as described in Figure 1. Tumors collected 2 h after the last treatments were processed for immunohistochemistry as previously described [12]. Representative images of tumor sections from vehicle- and drug-treated mice stained for p-Histone H3 Ser10, cleaved PARP, and CD31 are shown (A, B). Bars: 25 μM. RNA from indicated tumors was extracted and subjected to qRT-PCR. Representative ethidium bromide-stained gels for HCC21-0208 (C) HCC01-0909 (D), HCC13-0212 (E), and HCC10-0505 (F) are shown. Supplementary Data 5. Quantification of mean microvessel density, lectin-positive blood vessels, p-Histone 3 Ser10, and cleaved PARP-positive cells. Mice bearing high FGF19-expressing HCC09-0913 and high FGFR2/3-expressing HCC01-0909 xenografts were treated with 1) vehicle, 2) infigratinib, 3) FGF401, 4) infigratinib/FGF401 for 10 days as described in Figure 1. Vehicle- and drug-treated mice were perfused with biotinylated Lycopersicon Esculentum (tomato) lectin as described [12]. Tumors collected 2 h after the last treatments were processed for immunohistochemistry as previously described [12]. Sections (5-µm) were immunostained with cluster of differentiation 31, lectin, p-histone 3 Ser10, and cleaved PARP antibodies to assess microvessel density, lectin, cell proliferation, and apoptosis, respectively. The number of p-histone 3 Ser10 and cleaved PARP-positive cells, among at least 500 cells counted per region, was determined and plotted as the mean number of positive cells per 1000 cells ± SE. For the quantification of the mean lectin-positive blood vessels and microvessel density ± SE in each section, five random 0.159 mm2 fields at a magnification of x100 were observed for each tumor. Images were captured via Olympus BX60 light microscopy. Differences in mean microvessel density, lectin-positive blood vessels, p-histone 3 Ser10, and cleaved PARP-positive cells were compared using one-way analysis of variance followed by the Tukey–Kramer method post hoc test. *p < 0.05; **p < 0.01; ***p < 0.001. SE, standard error of the mean. Supplementary Data 6. Effects of infigratinib/FGF401 on the FGFR and its downstream targets in low FGFR and null FGF19 HCC10-0505. Mice bearing null FGF19 and low FGFR-expressing HCC10-0505 tumors were treated with 1) vehicle, 2) infigratinib once daily, 3) FGF401, and 4) infigratinib/FGF401 as described in Figure 1 for 4 days. Tumors were collected 2 h after the last dose of drugs. Tumor lysates were subjected to Western blot analysis as previously described [12]. Blots were incubated with indicated antibodies. Representative blots are shown. Supplementary Data 7. The effects of infigratinib/FGF401 on overall survival of mice bearing orthotopic HCC. Mice bearing HCC13-0212 and HCC26-0808A tumors (n = 10 per group) were treated with 1) vehicle, 2) infigratinib, 3) FGF401, or 4) infigratinib/FGF401 for 28 days. Treatments started when the tumors reached approximately 100–150 mm3. Representative Kaplan–Meier survival analyses for HCC13-0212 and HCC26-0808A are shown. Supplementary Data 8. Infigratinib/FGF401 improves antitumor immunity. HCC01-0909 tumors were subcutaneously implanted in CD34+ hu-NSG humanized mice and treated with vehicle, infigratinib, FGF401, and combined infigratinib and FGF401 as described in Figure 6 for 11 days. Sections were immunostained with antibodies against CD45, CD11c, CD8α, CD68, granzyme B, CD20, and CD4. Six random 0.159 mm2 fields at a magnification of x100 were captured using an Olympus BX60 light microscope and staining-positive cells were quantified. Representative images of tumor sections stained with CD45, CD11c, CD8α, CD68, granzyme B, CD20, and CD4 antibodies are shown (A). Magnification: 100x. For the quantification of mean CD45, CD4, CD8α, CD68, CD11c, and granzyme B cells, five random fields at a magnification of 100x were selected for each section. The number of CD45-, CD4-, CD8α-, CD68-, CD11c-, and granzyme B-positive cells per field was counted and expressed as mean ± SE (B). Differences in the means of staining-positive cells were compared. * p < 0.05; ** p < 0.01; *** p < 0.001; n.s. not signification. SE, standard error of the mean. Supplementary Data 9. Infigratinib/FGF401 improves antitumor immunity. HCC01-0909 tumors were subcutaneously implanted in CD34+ hu-NSG humanized mice and treated with vehicle, infigratinib, FGF401, and combined infigratinib and FGF401 as described in Figure 6 for 11 days. Sections were immunostained with antibodies against VISTA and PD-L1. Quantification of mean of VISTA and PD-L1 was performed as described in Supplementary Data 8 and results expressed as mean ± SE. Differences in the means of staining-positive cells were compared. * p < 0.05. SE, standard error of the mean. Supplementary Data 10. Infigratinib plus FGF401 was additive or synergistic. A graph of equally effective dose pairs for a single effect level (isobologram) was constructed using the median effect dose for infigratinib, FGF401, and infigratinib plus FGF401 on HCC01-0909 and HCC29-1104 tumors as described by Tallarida [29]. Point A represents the dosage of infigratinib alone (mg/kg) to achieve approximately 50% tumor growth inhibition and point B symbolizes the required FGF401 dosage alone (mg/kg twice daily) to obtain approximately 50% of tumor growth inhibition. Any dose combination of the two drugs that falls on or close to the straight line that links point A to B is defined as additive. Any dose combination that falls to the left of the straight line is defined as superadditive/synergistic. Note that all dose pairs of high FGFR2/3-expressing HCC01-0909 or high FGF19-expressing HCC29-1104 have combination indices (CI) that are less than 1 and fall on the left side of the straight line. These are categorized as synergistic. (PDF 11928 KB)
Rights and permissions
About this article
Cite this article
Tai, D.W.M., Le, T.B.U., Prawira, A. et al. Targeted inhibition of FGF19/FGFR cascade improves antitumor immunity and response rate in hepatocellular carcinoma. Hepatol Int 15, 1236–1246 (2021). https://doi.org/10.1007/s12072-021-10212-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10212-8