Abstract
Minocycline has beneficial effects in early brain injury (EBI) following subarachnoid hemorrhage (SAH); however, the molecular mechanisms underlying these effects have not been clearly identified. This study was undertaken to determine the influence of minocycline on inflammation and neural apoptosis and the possible mechanisms of these effects in early brain injury following subarachnoid hemorrhage. SAH was induced by the filament perforation model of SAH in male Sprague–Dawley rats. Minocycline or vehicle was given via an intraperitoneal injection 1 h after SAH induction. Minocycline treatment markedly attenuated brain edema secondary to blood-brain barrier (BBB) dysfunction by inhibiting NLRP3 inflammasome activation, which controls the maturation and release of pro-inflammatory cytokines, especially interleukin-1β (IL-1β). Minocycline treatment also markedly reduced the number of terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL)-positive cells. To further identify the potential mechanisms, we demonstrated that minocycline increased Bcl2 expression and reduced the protein expression of P53, Bax, and cleaved caspase-3. In addition, minocycline reduced the cortical levels of reactive oxygen species (ROS), which are closely related to both NLRP3 inflammasome and P53 expression. Minocycline protects against NLRP3 inflammasome-induced inflammation and P53-associated apoptosis in early brain injury following SAH. Minocycline’s anti-inflammatory and anti-apoptotic effect may involve the reduction of ROS. Minocycline treatment may exhibit important clinical potentials in the management of SAH.







Similar content being viewed by others
References
Ho HW, Batjer HH (1997) Aneurysmal subarachnoid hemorrhage: pathophysiology and sequelae. Cerebrovascular disease. Lippincott-Raven Publishers, Philadelphia
Sehba FA, Hou J, Pluta RM, Zhang JH (2012) The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol 97(1):14–37. doi:10.1016/j.pneurobio.2012.02.003
Caner B, Hou J, Altay O, Fujii M, Zhang JH (2012) Transition of research focus from vasospasm to early brain injury after subarachnoid hemorrhage. J Neurochem 123:12–21. doi:10.1111/j.1471-4159.2012.07939.x
Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, Zhang J, Tang J et al (2014) Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol 115C:64–91. doi:10.1016/j.pneurobio.2013.09.002
Dumont AS, Dumont RJ, Chow MM, Lin CL, Calisaneller T, Ley KF, Kassell NF, Lee KS (2003) Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery 53(1):123–133, discussion 133-125
Maddahi A, Povlsen GK, Edvinsson L (2012) Regulation of enhanced cerebrovascular expression of proinflammatory mediators in experimental subarachnoid hemorrhage via the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway. J Neuroinflammation 9:274. doi:10.1186/1742-2094-9-274
You WC, Wang CX, Pan YX, Zhang X, Zhou XM, Zhang XS, Shi JX, Zhou ML (2013) Activation of nuclear factor-kappaB in the brain after experimental subarachnoid hemorrhage and its potential role in delayed brain injury. PLoS One 8(3), e60290. doi:10.1371/journal.pone.0060290
Wang Z, Wu L, You W, Ji C, Chen G (2013) Melatonin alleviates secondary brain damage and neurobehavioral dysfunction after experimental subarachnoid hemorrhage: possible involvement of TLR4-mediated inflammatory pathway. J Pineal Res 55(4):399–408. doi:10.1111/jpi.12087
Chen S, Ma Q, Krafft PR, Hu Q, Rolland W II, Sherchan P, Zhang J, Tang J et al (2013) P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiol Dis 58:296–307. doi:10.1016/j.nbd.2013.06.011
Ostrowski RP, Colohan AR, Zhang JH (2006) Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res 28(4):399–414. doi:10.1179/016164106x115008
Hasegawa Y, Suzuki H, Sozen T, Altay O, Zhang JH (2011) Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage. Acta Neurochir Suppl 110(Pt 1):43–48. doi:10.1007/978-3-7091-0353-1_8
Cahill J, Calvert JW, Solaroglu I, Zhang JH (2006) Vasospasm and p53-induced apoptosis in an experimental model of subarachnoid hemorrhage. Stroke 37(7):1868–1874. doi:10.1161/01.str.0000226995.27230.96
Zhou C, Yamaguchi M, Colohan AR, Zhang JH (2005) Role of p53 and apoptosis in cerebral vasospasm after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 25(5):572–582. doi:10.1038/sj.jcbfm.9600069
Cahill J, Calvert JW, Marcantonio S, Zhang JH (2007) p53 may play an orchestrating role in apoptotic cell death after experimental subarachnoid hemorrhage. Neurosurgery 60(3):531–545. doi:10.1227/01.neu.0000249287.99878.9b, discussion 545
Cahill J, Calvert JW, Zhang JH (2006) Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 26(11):1341–1353. doi:10.1038/sj.jcbfm.9600283
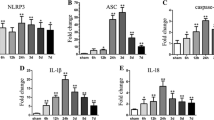
Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J (2014) NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol 75(2):209–219. doi:10.1002/ana.24070
Zong WX, Thompson CB (2006) Necrotic death as a cell fate. Genes Dev 20(1):1–15. doi:10.1101/gad.1376506
Garrido-Mesa N, Zarzuelo A, Galvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169(2):337–352. doi:10.1111/bph.12139
Koistinaho M, Malm TM, Kettunen MI, Goldsteins G, Starckx S, Kauppinen RA, Opdenakker G, Koistinaho J (2005) Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab 25(4):460–467. doi:10.1038/sj.jcbfm.9600040
Sanchez Mejia RO, Ona VO, Li M, Friedlander RM (2001) Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery 48(6):1393–1399, discussion 1399-1401
Abdel-Salam OM (2008) Drugs used to treat Parkinson's disease, present status and future directions. CNS Neurol Disord Drug Targets 7(4):321–342
Guo ZD, Wu HT, Sun XC, Zhang XD, Zhang JH (2011) Protection of minocycline on early brain injury after subarachnoid hemorrhage in rats. Acta Neurochir Suppl 110(Pt 1):71–74. doi:10.1007/978-3-7091-0353-1_13
Sherchan P, Lekic T, Suzuki H, Hasegawa Y, Rolland W, Duris K, Zhan Y, Tang J et al (2011) Minocycline improves functional outcomes, memory deficits, and histopathology after endovascular perforation-induced subarachnoid hemorrhage in rats. J Neurotrauma 28(12):2503–2512. doi:10.1089/neu.2011.1864
Chen J, Wang L, Wu C, Hu Q, Gu C, Yan F, Li J, Yan W et al (2014) Melatonin-enhanced autophagy protects against neural apoptosis via a mitochondrial pathway in early brain injury following a subarachnoid hemorrhage. J Pineal Res 56(1):12–19. doi:10.1111/jpi.12086
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 26(4):627–634, discussion 635
Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 167(2):327–334. doi:10.1016/j.jneumeth.2007.08.004
Topkoru BC, Altay O, Duris K, Krafft PR, Yan J, Zhang JH (2013) Nasal administration of recombinant osteopontin attenuates early brain injury after subarachnoid hemorrhage. Stroke 44(11):3189–3194. doi:10.1161/strokeaha.113.001574
Yan F, Hu Q, Chen J, Wu C, Gu C, Chen G (2013) Progesterone attenuates early brain injury after subarachnoid hemorrhage in rats. Neurosci Lett 543:163–167. doi:10.1016/j.neulet.2013.03.005
Zetterling M, Hallberg L, Hillered L, Karlsson T, Enblad P, Ronne Engstrom E (2010) Brain energy metabolism in patients with spontaneous subarachnoid hemorrhage and global cerebral edema. Neurosurgery 66(6):1102–1110. doi:10.1227/01.neu.0000370893.04586.73
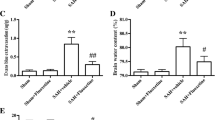
Altay O, Suzuki H, Hasegawa Y, Caner B, Krafft PR, Fujii M, Tang J, Zhang JH (2012) Isoflurane attenuates blood-brain barrier disruption in ipsilateral hemisphere after subarachnoid hemorrhage in mice. Stroke 43(9):2513–2516. doi:10.1161/strokeaha.112.661728
Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA (2002) Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke 33(5):1225–1232
Li Z, Liang G, Ma T, Li J, Wang P, Liu L, Yu B, Liu Y et al (2014) Blood-brain barrier permeability change and regulation mechanism after subarachnoid hemorrhage. Metab Brain Dis. doi:10.1007/s11011-014-9609-1
Sehba FA, Mostafa G, Knopman J, Friedrich V, Bederson JB (2004) Acute alterations in microvascular basal lamina after subarachnoid hemorrhage. J Neurosurg 101(4):633–640. doi:10.3171/jns.2004.101.4.0633
Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 21(19):7724–7732
Bauer AT, Burgers HF, Rabie T, Marti HH (2010) Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab 30(4):837–848. doi:10.1038/jcbfm.2009.248
Sozen T, Tsuchiyama R, Hasegawa Y, Suzuki H, Jadhav V, Nishizawa S, Zhang JH (2009) Role of interleukin-1 beta in early brain injury after subarachnoid hemorrhage in mice. Stroke 40(7):2519–2525. doi:10.1161/strokeaha.109.549592
Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H et al (2013) Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4, e525. doi:10.1038/cddis.2013.54
Jha S, Srivastava SY, Brickey WJ, Iocca H, Toews A, Morrison JP, Chen VS, Gris D et al (2010) The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci 30(47):15811–15820. doi:10.1523/jneurosci.4088-10.2010
Matsukawa N, Yasuhara T, Hara K, Xu L, Maki M, Yu G, Kaneko Y, Ojika K et al (2009) Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci 10:126. doi:10.1186/1471-2202-10-126
Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Park CH, Jeong YH, Yoo J et al (2007) Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer's disease models. Neuropsychopharmacology 32(11):2393–2404. doi:10.1038/sj.npp.1301377
Leker RR, Aharonowiz M, Greig NH, Ovadia H (2004) The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin alpha. Exp Neurol 187(2):478–486. doi:10.1016/j.expneurol.2004.01.030
Mendelow AD (1988) Pathophysiology of delayed ischaemic dysfunction after subarachnoid haemorrhage: experimental and clinical data. Acta Neurochir Suppl (Wien) 45:7–10
Antonsson B, Martinou JC (2000) The Bcl-2 protein family. Exp Cell Res 256(1):50–57. doi:10.1006/excr.2000.4839
Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC et al (1999) Ordering the cytochrome c-initiated caspase cascade: Hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol 144(2):281–292. doi:10.1083/jcb.144.2.281
Zheng Y, Xu L, Yin J, Zhong Z, Fan H, Li X, Chang Q (2013) Effect of minocycline on cerebral ischemia-reperfusion injury. Neural Regen Res 8(10):900–908. doi:10.3969/j.issn.1673-5374.2013.10.004
Hayakawa K, Mishima K, Nozako M, Hazekawa M, Mishima S, Fujioka M, Orito K, Egashira N et al (2008) Delayed treatment with minocycline ameliorates neurologic impairment through activated microglia expressing a high-mobility group box1-inhibiting mechanism. Stroke 39(3):951–958. doi:10.1161/strokeaha.107.495820
Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC (2006) Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci 7:56. doi:10.1186/1471-2202-7-56
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69. doi:10.1038/nrn2038
Acknowledgments
This study was supported by the Scientific and Technological Project of Zhejiang Province (2013C33138) and the Qianjiang rencai project (2013R10029).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jianru Li, Jingsen Chen and Hangbo Mo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, J., Chen, J., Mo, H. et al. Minocycline Protects Against NLRP3 Inflammasome-Induced Inflammation and P53-Associated Apoptosis in Early Brain Injury After Subarachnoid Hemorrhage. Mol Neurobiol 53, 2668–2678 (2016). https://doi.org/10.1007/s12035-015-9318-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9318-8