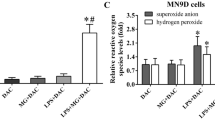
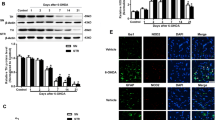
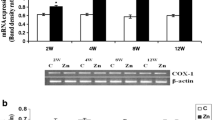
Abstract
Parkinson’s disease (PD) patients have excessive iron depositions in substantia nigra (SN). Neuroinflammation characterized by microglial activation is pivotal for dopaminergic neurodegeneration in PD. However, the role and mechanism of microglial activation in iron-induced dopaminergic neurodegeneration in SN remain unclear yet. This study aimed to investigate the role and mechanism of microglial β-nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2) activation in iron-induced selective and progressive dopaminergic neurodegeneration. Multiple primary midbrain cultures from rat, NOX2+/+ and NOX2−/− mice were used. Dopaminergic neurons, total neurons, and microglia were visualized by immunostainings. Cell viability was measured by MTT assay. Superoxide (O2 ·−) and intracellular reactive oxygen species (iROS) were determined by measuring SOD-inhibitable reduction of tetrazolium salt WST-1 and DCFH-DA assay. mRNA and protein were detected by real-time PCR and Western blot. Iron induces selective and progressive dopaminergic neurotoxicity in rat neuron–microglia–astroglia cultures and microglial activation potentiates the neurotoxicity. Activated microglia produce a magnitude of O2 ·− and iROS, and display morphological alteration. NOX2 inhibitor diphenylene iodonium protects against iron-elicited dopaminergic neurotoxicity through decreasing microglial O2 ·− generation, and NOX2−/− mice are resistant to the neurotoxicity by reducing microglial O2 ·− production, indicating that iron-elicited dopaminergic neurotoxicity is dependent of NOX2, a O2 ·−-generating enzyme. NOX2 activation is indicated by the increased mRNA and protein levels of subunits P47 and gp91. Molecules relevant to NOX2 activation include PKC-σ, P38, ERK1/2, JNK, and NF-КBP65 as their mRNA and protein levels are enhanced by NOX2 activation. Iron causes selective and progressive dopaminergic neurodegeneration, and microglial NOX2 activation potentiates the neurotoxicity. PKC-σ, P38, ERK1/2, JNK, and NF-КBP65 are the potential molecules relevant to microglial NOX2 activation.







Similar content being viewed by others
References
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69. doi:10.1038/nrn2038
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291, PMID: 3399080
Zhang W, Wang T, Qin L, Gao HM, Wilson B, Ali SF et al (2004) Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: role of NADPH oxidase. FASEB J 18:589–591. doi:10.1096/fj.03-0983fje
Jung BD, Shin EJ, Nguyen XK, Jin CH, Bach JH, Park SJ et al (2010) Potentiation of methamphetamine neurotoxicity by intrastriatal lipopolysaccharide administration. Neurochem Int 56:229–244. doi:10.1016/j.neuint.2009.10.005
Gao HM, Hong JS, Zhang W, Liu B (2003) Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. J Neurosci 23:1228–1236, PMID: 12598611
Miller RL, Sun GY, Sun AY (2007) Cytotoxicity of paraquat in microglial cells: involvement of PKCdelta- and ERK1/2-dependent NADPH oxidase. Brain Res 1167:129–139. doi:10.1016/j.brainres.2007.06.046
Zecca L, Zucca FA, Albertini A, Rizzio E, Fariello RG (2006) A proposed dual role of neuromelanin in the pathogenesis of Parkinson’s disease. Neurology 67:S8–S11. doi:10.1212/WNL.67.7_suppl_2.S8
Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML et al (2005) Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 19:533–542. doi:10.1096/fj.04-2751com
Zhang W, Dallas S, Zhang D, Guo JP, Pang H, Wilson B et al (2007) Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia 55:1178–1188. doi:10.1002/glia.20532
Bernstein AI, Garrison SP, Zambetti GP, O'Malley KL (2011) 6-OHDA generated ROS induces DNA damage and p53- and PUMA-dependent cell death. Mol Neurodegener 6:2. doi:10.1186/1750-1326-6-2
Cho Y, Son HJ, Kim EM, Choi JH, Kim ST, Ji IJ et al (2009) Doxycycline is neuroprotective against nigral dopaminergic degeneration by a dual mechanism involving MMP-3. Neurotox Res 16:361–371. doi:10.1007/s12640-009-9078-1
Levesque S, Wilson B, Gregoria V, Thorpe LB, Dallas S, Polikov VS et al (2010) Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity. Brain 133:808–821. doi:10.1093/brain/awp333
Block ML, Wu X, Pei Z, Li G, Wang T, Qin L et al (2004) Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J 18:1618–1620. doi:10.1096/fj.04-1945fje
Arreguin S, Nelson P, Padway S, Shirazi M, Pierpont C (2009) Dopamine complexes of iron in the etiology and pathogenesis of Parkinson’s disease. J Inorg Biochem 103:87–93. doi:10.1016/j.jinorgbio.2008.09.007
Berg D, Hochstrasser H (2006) Iron metabolism in Parkinsonian syndromes. Mov Disord 21:1299–1310. doi:10.1002/mds.21020
Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE et al (1991) Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114(Pt 4):1953–1975. doi:10.1093/brain/114.4.1953
Powers KM, Smith-Weller T, Franklin GM, Longstreth WT Jr, Swanson PD, Checkoway H (2009) Dietary fats cholesterol and iron as risk factors for Parkinson’s disease. Parkinsonism Relat Disord 15:47–52. doi:10.1016/j.parkreldis.2008.03.002
Barbeito AG, Garringer HJ, Baraibar MA, Gao X, Arredondo M, Nunez MT et al (2009) Abnormal iron metabolism and oxidative stress in mice expressing a mutant form of the ferritin light polypeptide gene. J Neurochem 109:1078. doi:10.1111/j.1471-4159.2009.06028.x
Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT et al (2005) Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57:176–179. doi:10.1002/ana.20369
Riederer P, Rausch WD, Schmidt B, Kruzik P, Konradi C, Sofic E et al (1988) Biochemical fundamentals of Parkinson’s disease. Mt Sinai J Med 55:21–28, PMID: 3279302
Hare D, Ayton S, Bush A, Lei P (2013). A delicate balance: iron metabolism and diseases of the brain. Front Aging Neurosci. 5:34. doi: 10.3389/fnagi.2013.00034. eCollection: 2013.
Dexter DT, Carayon A, Vidailhet M, Ruberg M, Agid F, Agid Y et al (1990) Decreased ferritin levels in brain in Parkinson’s disease. J Neurochem 55:16–20. doi:10.1111/j.1471-4159.1990.tb08814.x
Werner CJ, Heyny-Von Haussen R, Mall G, Wolf S (2008) Proteome analysis of human substantia nigra in Parkinson’s disease. Proteome Sci 6:8. doi:10.1186/1477-5956-6-8
Zhang X, Dong F, Ren J, Driscoll MJ, Culver B (2005) High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol 191:318–325. doi:10.1016/j.expneurol.2004.10.011
Kelleher P, Pacheco K, Newman LS (2000) Inorganic dust pneumonias: the metal-related parenchymal disorders. Environ Health Perspect 108(Suppl 4):685–696, PMID: 10931787
Zhang W, Phillips K, Wielgus AR, Liu J, Albertini A, Zucca FA et al (2011) Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson's disease. Neurotox Res 19:63–72. doi:10.1007/s12640-009-9140-z
Aisen P, Enns C, Wessling-Resnick M (2001) Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol 33:940–959. doi:10.1016/S1357-2725(01) 00063-2
Moos T, Morgan EH (2004) The metabolism of neuronal iron and its pathogenic role in neurological disease: review. Ann N Y Acad Sci 1012:14–26. doi:10.1196/annals.1306.002
Leitner DF, Connor JR (2012) Functional roles of transferrin in the brain. Biochim Biophys Acta 1820:393–402. doi:10.1016/j.bbagen.2011.10.016
Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S et al (2005) The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1:191–200. doi:10.1016/j.cmet.2005.01.003
Abboud S, Haile DJ (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275:19906–19912. doi:10.1074/jbc.M000713200
Dringen R, Bishop GM, Koeppe M, Dang TN, Robinson SR (2007) The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem Res 32:1884–1890. doi:10.1007/s11064-007-9375-0
Lowe JS, Leigh N (2002). Disorders of movement and system degenerations. In Graham DI, Lantos PL, editors. Greenfield’s Neuropathology. 7th ed. London: Arnold. 2: 325–430.
Yamada T, McGeer PL, McGeer EG (1992) Lewy bodies in Parkinson’s disease are recognized by antibodies to complement proteins. Acta Neuropathol 84:100–104, PMID: 1502878
Castellani RJ, Siedlak SL, Perry G, Smith MA (2000) Sequestration of iron by Lewy bodies in Parkinson’s disease. Acta Neuropathol 100:111–114. doi:10.1007/s004010050001
Lv Z, Jiang H, Xu H, Song N, Xie J (2011) Increased iron levels correlate with the selective nigral dopaminergic neuron degeneration in Parkinson's disease. J Neural Transm 118:361–369. doi:10.1007/s00702-010-0434-3
Chew KC, Ang ET, Tai YK, Tsang F, Lo SQ, Ong E et al (2011) Enhanced autophagy from chronic toxicity of iron and mutant A53T alpha-synuclein: implications for neuronal cell death in Parkinson disease. J Biol Chem 286:33380–33389. doi:10.1074/jbc.M111.268409
Wypijewska A, Galazka-Friedman J, Bauminger ER, Wszolek ZK, Schweitzer KJ, Dickson DW et al (2010) Iron and reactive oxygen species activity in parkinsonian substantia nigra. Parkinsonism Relat Disord 16:329–333. doi:10.1016/j.parkreldis.2010.02.007
Dutta G, Zhang P, Liu B (2008) The lipopolysaccharide Parkinson’s disease animal model: mechanistic studies and drug discovery. Fundam Clin Pharmacol 22:453–464. doi:10.1111/j.1472-8206.2008.00616.x
Tansey MG, Goldberg MS (2010) Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 37:510–518. doi:10.1016/j.nbd.2009.11.004
Infanger DW, Sharma RV, Davisson RL (2006) NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal 8:1583–1596. doi:10.1089/ars.2006.8.1583
Stewart VC, Heales SJ (2003) Nitric oxide-induced mitochondrial dysfunction: implications for neurodegeneration. Free Radic Biol Med 34:287–303. doi:10.1016/S0891-5849(02)01327-8
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS et al (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55:453–462. doi:10.1002/glia.20467
McGeer PL, McGeer EG (2008) Glial reactions in Parkinson's disease. Mov Disord 23:474–483. doi:10.1002/mds.21751
Ouchi Y, Yagi S, Yokokura M, Sakamoto M (2009) Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord 15(Suppl 3):S200–S204. doi:10.1016/S1353-8020(09)70814-4
Block ML (2008) NADPH oxidase as a therapeutic target in Alzheimer's disease. BMC Neurosci 9(Suppl 2):S8. doi:10.1186/1471-2202-9-S2-S8
Tammariello SP, Quinn MT, Estus S (2000) NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci 20:RC53, PMID: 10627630
Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B et al (2004) NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem 279:1415–1421. doi:10.1074/jbc.M307657200
Casarejos MJ, Menendez J, Solano RM, Rodriguez-Navarro JA, de Yebenes JG, Mena MA (2006) Susceptibility to rotenone is increased in neurons from parkin null mice and is reduced by minocycline. J Neurochem 97:934–946. doi:10.1111/j.1471-4159.2006.03777.x
Sorce S, Krause KH (2009) NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal 11:2481–2504. doi:10.1089/ARS.2009.2578
Miller RL, James-Kracke M, Sun GY, Sun AY (2009) Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem Res 34:55–65. doi:10.1007/s11064-008-9656-2
Zhao X, Xu B, Bhattacharjee A, Oldfield CM, Wientjes FB, Feldman GM et al (2005) Protein kinase Cdelta regulates p67phox phosphorylation in human monocytes. J Leukoc Biol 77:414–420. doi:10.1189/jlb.0504284
Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q et al (2004) Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol 173:5730–5738, PMID: 15494525
Min KJ, Pyo HK, Yang MS, Ji KA, Jou I, Joe EH (2004) Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia 48:197–206. doi:10.1002/glia.20069
Murphy LO, Blenis J (2006) MAPK signal specificity: the right place at the right time. Trends Biochem Sci 31:268–275. doi:10.1016/j.tibs.2006.03.009
Bardwell L (2006) Mechanisms of MAPK signalling specificity. Biochem Soc Trans 34:837–841. doi:10.1042/BST0340837
Flohe L, Brigelius-Flohe R, Saliou C, Traber MG, Packer L (1997) Redox regulation of NF-kappa B activation. Free Radic Biol Med 22:1115–1126. doi:10.1016/S0891-5849(96)00501-1
Yang S, Yang J, Yang Z, Chen P, Fraser A, Zhang W et al (2006) Pituitary adenylate cyclase-activating polypeptide (PACAP) 38 and PACAP4-6 are neuroprotective through inhibition of NADPH oxidase: potent regulators of microglia-mediated oxidative stress. J Pharmacol Exp Ther 319:595–603. doi:10.1124/jpet.106.102236
Acknowledgments
This work is supported by the National Basic Research Program of China (2011CB504100); the National Natural Science Foundation of China (81071015, 30770745, and 81030062), the Natural Science Foundation of Beijing, China (7082032), High Level Technical Personnel Training Project of Beijing Health System, China (2009-3-26), Excellent Personnel Training Project of Beijing, China (20071D0300400076), Capital Clinical Characteristic Application Research (Z121107001012161), Important National Science and Technology Specific Projects ( 2011ZX09102-003-01), Key Project of Beijing Natural Science Foundation (kz200910025001), and Basic-Clinical Research Cooperation Funding of Capital Medical University (10JL49). We thank Dr. Yang Du for her efforts of editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 57.5 KB)
Rights and permissions
About this article
Cite this article
Zhang, W., Yan, Zf., Gao, Jh. et al. Role and Mechanism of Microglial Activation in Iron-Induced Selective and Progressive Dopaminergic Neurodegeneration. Mol Neurobiol 49, 1153–1165 (2014). https://doi.org/10.1007/s12035-013-8586-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8586-4