Abstract
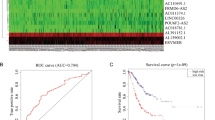
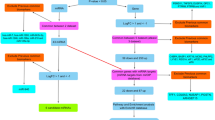
Altered expressions of microRNAs (miRNAs) are reported in pancreatic cancer and associate with cancer pathogenesis, apoptosis, and cell growth, thereby functioning as either tumor suppressors or oncogenes. However, the majority of studies focus on defining the regulatory functions of miRNAs, whereas few investigations are directed toward assessing how the miRNA themselves are transcriptionally regulated. In this study, integration of published multi-level expression data and bioinformatics computational approach was used to predict two regulation mechanisms: transcription factors (TF)–miRNA–mRNA regulation and long non-coding RNA(lncRNA)–miRNA–mRNA regulation. To identify differentially expressed mRNAs, miRNAs, and lncRNAs, we integrated microarray expression data in pancreatic cancer tissues and normal tissues. Combination of differentially expressed mRNAs and miRNAs with miRNA–mRNA interactions based on crosslinking and immunoprecipitation followed by high-throughput sequencing (CLIP-Seq) data from StarBas, we constructed miRNA–mRNA regulatory network. Then we constructed two regulatory networks including TF–miRNA–mRNA and lncRNA–miRNA–mRNA based on chromatin immunoprecipitation followed by high-throughput sequencing (ChIP-Seq) data from ChIPBase and CLIP-Seq data. A total of 4385 mRNAs, 500 miRNAs, and 21 lncRNAs were differentially expressed, of which, 18 mRNAs and 54 miRNAs are with high confidence. In miRNA–mRNA regulatory network, interrelated miRNAs target 1701 differentially regulated mRNAs. By constructing regulatory network, 19miRNAs including hsa-miR-137, hsa-miR-206, hsa-miR-429, hsa-miR-320d, and hsa-miR-320c are predicted to participate in lncRNA–miRNA–mRNA regulation. Furthermore, 8 miRNAs including hsa-mir-137, hsa-mir-206, hsa-mir-429, hsa-mir-375, hsa-mir-326, hsa-mir-217, hsa-mir-301b, and hsa-mir-184 are predicted to participate in TF–miRNA–mRNA regulation. In an integrated data analysis, we reveal large-scale effects of interrelated miRNAs and provide a model for predicting the mechanism of miRNAs disorder. Our study provides a new insight into understanding the transcriptional regulation of pancreatic cancer.





Similar content being viewed by others
References
Ascano, M., et al. (2012). Identification of RNA–protein interaction networks using PAR-CLIP. Wiley Interdisciplinary Reviews: Rna, 3(2), 159–177.
Cline, M. S., et al. (2007). Integration of biological networks and gene expression data using Cytoscape. Nature Protocols, 2(10), 2366–2382.
Darnell, R. B. (2010). HITS-CLIP: panoramic views of protein–RNA regulation in living cells. Wiley Interdisciplinary Reviews: Rna, 1(2), 266–286.
Dayem, U. A., et al. (2014). The pancreatic expression database: Recent extensions and updates. Nucleic Acids Research, 42, D944–D949.
Donahue, T. R., et al. (2012). Integrative survival-based molecular profiling of human pancreatic cancer. Clinical Cancer Research, 18(5), 1352–1363.
Farnham, P. J. (2009). Insights from genomic profiling of transcription factors. Nature Reviews Genetics, 10(9), 605–616.
Frampton, A. E., et al. (2014). MicroRNAs cooperatively inhibit a network of tumor suppressor genes to promote pancreatic tumor growth and progression. Gastroenterology, 146(1), 268–277.
Huang, D. W., Sherman, B. T., & Lempicki, R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols, 4(1), 44–57.
Jalali, S., et al. (2013). Systematic transcriptome wide analysis of lncRNA–miRNA interactions. PLoS One, 8(2), e53823.
Kefas, B., et al. (2010). Pyruvate kinase M2 is a target of the tumor-suppressive microRNA-326 and regulates the survival of glioma cells. Neuro Oncology, 12(11), 1102–1112.
Kim, K., et al. (2013). HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene, 32(13), 1616–1625.
Konig, J., et al. (2011). Protein–RNA interactions: new genomic technologies and perspectives. Nature Reviews Genetics, 13(2), 77–83.
Li, J. H., et al. (2014). starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Research, 42(1), D92–D97.
Liu, J. H., et al. (2014). Expression and prognostic significance of lncRNA MALAT1 in pancreatic cancer tissues. Asian Pacific Journal of Cancer Prevention, 15(7), 2971–2977.
Ma, C., et al. (2014). H19 promotes pancreatic cancer metastasis by derepressing let-7′s suppression on its target HMGA2-mediated EMT. Tumor Biology, 1–7.
Panzitt, K., et al. (2007). Characterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNA. Gastroenterology, 132(1), 330–342.
Park, P. J. (2009). ChIP-seq: advantages and challenges of a maturing technology. Nature Reviews Genetics, 10(10), 669–680.
Pepke, S., Wold, B., & Mortazavi, A. (2009). Computation for ChIP-seq and RNA-seq studies. Nature Methods, 6(11), S22–S32.
Tahira, A. C., et al. (2011). Long noncoding intronic RNAs are differentially expressed in primary and metastatic pancreatic cancer. Mol Cancer, 10, 141.
Wang, J., et al. (2010). CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Research, 38(16), 5366–5383.
Wang, G., et al. (2013). MicroRNA 125 represses nonsense-mediated mRNA decay by regulating SMG1 expression. Biochemical and Biophysical Research Communications, 435(1), 16–20.
Xu, H., et al. (2010). Liver-enriched transcription factors regulate microRNA-122 that targets CUTL1 during liver development. Hepatology, 52(4), 1431–1442.
Yang, J. H., et al. (2011). starBase: A database for exploring microRNA-mRNA interaction maps from argonaute CLIP-Seq and degradome-seq data. Nucleic Acids Research, 39, D202–D209.
Yang, J. H., et al. (2013). ChIPBase: A database for decoding the transcriptional regulation of long non-coding RNA and microRNA genes from ChIP-Seq data. Nucleic Acids Research, 41, D177–D187.
Zhao, Y., & Simon, R. (2008). BRB-ArrayTools Data Archive for human cancer gene expression: A unique and efficient data sharing resource. Cancer Informatics, 6, 9–15.
Zhao, W. G., et al. (2010). The miR-217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis, 31(10), 1726–1733.
Acknowledgments
This study was supported by The research Special Fund For public welfare industry of health (No. 201202007), and Fund for National key specialty construction of clinical project (General Surgery), and Fund from the Education Department of Zhejiang Province (No. Y201328225), and Fund from the Health Department of Zhejiang Province (No. 201484382), and National High Technology Research and Development Program of China (863 Program, No. 2012AA02A205), and the National Natural Science Foundation of China (No. J20121214), and the Financial Support of Science Technology Department of Zhejiang Province (No.2011C23088) and Medical Science Research Foundation of Health Bureau of Zhejiang Province (No. 2012KYB070).
Conflict of interest
We have no conflict of interest to declare and informed consent was obtained.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ye, S., Yang, L., Zhao, X. et al. Bioinformatics Method to Predict Two Regulation Mechanism: TF–miRNA–mRNA and lncRNA–miRNA–mRNA in Pancreatic Cancer. Cell Biochem Biophys 70, 1849–1858 (2014). https://doi.org/10.1007/s12013-014-0142-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0142-y