Abstract
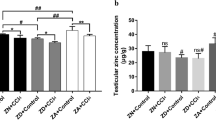
Despite the fact that iron represents a crucial element for the catalysis of many metabolic reactions, its accumulation in the cell leads to the production of reactive oxygen species (ROS), provoking pathological conditions such as cancer, cardiovascular diseases, diabetes, neurodegenerative diseases, and fertility. Thus, ROS are neutralized by the enzymatic antioxidant system for the purpose of protecting cells against any damage. Iron is a potential risk factor for male fertility. However, the mechanism of action of iron on the testicular antioxidant system at the gene and protein levels is not fully understood. Thus, the purpose of the current research was to ensure a better understanding of how the long-term iron treatment influences both gene expression and enzyme activities of the testicular antioxidant system in rat testis. The data of our study showed that a significant dose-dependent increase occurred in the iron level in rat testis. A reduction occurred in reduced glutathione (GSH) levels, which represent a marker of oxidative stress, along with long-term iron overload. The expression and activity of glucose 6-phosphate dehydrogenase (G6pd), glutathione reductase (Gr), glutathione peroxidase (Gpx), and glutathione S-transferases (Gst) were significantly affected by the presence of iron. The findings of the current research demonstrate that the long-term toxic dietary iron overload influences the gene expression and enzyme activity of the testicular antioxidant defense system, but the actual effect occurs at the protein level. This may modify the sperm function and dysfunction of the male reproductive system.



Similar content being viewed by others
References
Wang J, Pantopoulos K (2011) Regulation of cellular iron metabolism. Biochem J 434(3):365–381. https://doi.org/10.1042/BJ20101825
Steinbicker AU, Muckenthaler MU (2013) Out of balance--systemic iron homeostasis in iron-related disorders. Nutrients 5(8):3034–3061. https://doi.org/10.3390/nu5083034
De Freitas JM, Meneghini R (2001) Iron and its sensitive balance in the cell. Mutat Res 475(1-2):153–159
Weiss G, Ganz T, Goodnough LT (2019) Anemia of inflammation. Blood 133(1):40–50. https://doi.org/10.1182/blood-2018-06-856500
Budak H, Kocpinar EF, Gonul N, Ceylan H, Erol HS, Erdogan O (2014) Stimulation of gene expression and activity of antioxidant related enzyme in Sprague Dawley rat kidney induced by long-term iron toxicity. Comp Biochem Physiol Toxicol Pharmacol 166:44–50. https://doi.org/10.1016/j.cbpc.2014.07.002
Galaris D, Pantopoulos K (2008) Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit Rev Clin Lab Sci 45(1):1–23. https://doi.org/10.1080/10408360701713104
Imam MU, Zhang S, Ma J, Wang H, Wang F (2017) Antioxidants mediate both iron homeostasis and oxidative stress. Nutrients 9(7). https://doi.org/10.3390/nu9070671
Budak H, Gonul N, Ceylan H, Kocpinar EF (2014) Impact of long term Fe(3)(+) toxicity on expression of glutathione system in rat liver. Environ Toxicol Pharmacol 37(1):365–370. https://doi.org/10.1016/j.etap.2013.12.007
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5(1):9–19. https://doi.org/10.1097/WOX.0b013e3182439613
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):R453–R462. https://doi.org/10.1016/j.cub.2014.03.034
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74. https://doi.org/10.2174/157015909787602823
Karaman M, Budak H, Ciftci M (2018) Amoxicillin and gentamicin antibiotics treatment adversely influence the fertility and morphology through decreasing the Dazl gene expression level and increasing the oxidative stress. Arch Physiol Biochem:1–9. https://doi.org/10.1080/13813455.2018.1482354
Salmasi AH, Beheshtian A, Payabvash S, Demehri S, Ebrahimkhani MR, Karimzadegan M, Bahadori M, Pasalar P, Dehpour AR (2005) Effect of morphine on ischemia-reperfusion injury: experimental study in testicular torsion rat model. Urology 66(6):1338–1342. https://doi.org/10.1016/j.urology.2005.06.101
Aitken RJ (2004) Founders’ Lecture. Human spermatozoa: fruits of creation, seeds of doubt. Reprod Fertil Dev 16(7):655–664
Turner TT, Lysiak JJ (2008) Oxidative stress: a common factor in testicular dysfunction. J Androl 29(5):488–498. https://doi.org/10.2164/jandrol.108.005132
Aitken RJ, Roman SD (2008) Antioxidant systems and oxidative stress in the testes. Oxidative Med Cell Longev 1(1):15–24
Djuric A, Begic A, Gobeljic B, Stanojevic I, Ninkovic M, Vojvodic D, Pantelic A, Zebic G, Prokic V, Dejanovic B, Stojanovic I, Pavlica M, Djukic D, Saso L, Djurdjevic D, Pavlovic M, Topic A, Vujanovic D, Stevnovic I, Djukic M (2015) Oxidative stress, bioelements and androgen status in testes of rats subacutely exposed to cadmium. Food Chem Toxicol 86:25–33. https://doi.org/10.1016/j.fct.2015.09.004
Gabrielsen JS, Lamb DJ, Lipshultz LI (2018) Iron and a man’s reproductive health: the good, the bad, and the ugly. Curr Urol Rep 19(8):60. https://doi.org/10.1007/s11934-018-0808-x
Jadhav SH, Sarkar SN, Patil RD, Tripathi HC (2007) Effects of subchronic exposure via drinking water to a mixture of eight water-contaminating metals: a biochemical and histopathological study in male rats. Arch Environ Contam Toxicol 53(4):667–677. https://doi.org/10.1007/s00244-007-0031-0
Hirst JA, Howick J, Aronson JK, Roberts N, Perera R, Koshiaris C, Heneghan C (2014) The need for randomization in animal trials: an overview of systematic reviews. PloS One 9(6):e98856. https://doi.org/10.1371/journal.pone.0098856
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Yu SY, Sun L, Liu Z, Huang XY, Zuo LJ, Cao CJ, Zhang W, Wang XM (2013) Sleep disorders in Parkinson’s disease: clinical features, iron metabolism and related mechanism. PLoS One 8(12):e82924. https://doi.org/10.1371/journal.pone.0082924
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Akkemik E, Budak H, Ciftci M (2010) Effects of some drugs on human erythrocyte glucose 6-phosphate dehydrogenase: an in vitro study. J Enzyme Inhib Med Chem 25(6):871–875. https://doi.org/10.3109/14756360903489581
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Upham BL, Trosko JE (2009) Oxidative-dependent integration of signal transduction with intercellular gap junctional communication in the control of gene expression. Antioxid Redox Signal 11(2):297–307. https://doi.org/10.1089/ars.2008.2146
Salim S (2014) Oxidative stress and psychological disorders. Curr Neuropharmacol 12(2):140–147. https://doi.org/10.2174/1570159X11666131120230309
Filomeni G, De Zio D, Cecconi F (2015) Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ 22(3):377–388. https://doi.org/10.1038/cdd.2014.150
Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ (2008) Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab 93(8):3199–3207. https://doi.org/10.1210/jc.2007-2616
Valko M, Morris H, Cronin MT (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12(10):1161–1208
Choi JS, Koh IU, Lee HJ, Kim WH, Song J (2013) Effects of excess dietary iron and fat on glucose and lipid metabolism. J Nutr Biochem 24(9):1634–1644. https://doi.org/10.1016/j.jnutbio.2013.02.004
Lucesoli F, Caligiuri M, Roberti MF, Perazzo JC, Fraga CG (1999) Dose-dependent increase of oxidative damage in the testes of rats subjected to acute iron overload. Arch Biochem Biophys 372(1):37–43. https://doi.org/10.1006/abbi.1999.1476
Doreswamy K, Muralidhara (2005) Genotoxic consequences associated with oxidative damage in testis of mice subjected to iron intoxication. Toxicology 206(1):169–178
Gonul Baltaci N, Guler C, Ceylan H, Kalin SN, Adem S, Kocpinar EF, Erdogan O, Budak H (2018) In vitro and in vivo effects of iron on the expression and activity of glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, and glutathione reductase in rat spleen. J Biochem Mol Toxicol:e22229. https://doi.org/10.1002/jbt.22229
Franco R, Navarro G, Martinez-Pinilla E (2019) Antioxidant defense mechanisms in erythrocytes and in the central nervous system. Antioxidants 8(2). https://doi.org/10.3390/antiox8020046
Bozkurt A, Budak H, Erol HS, Can S, Mercantepe T, Akin Y, Ozbey I, Cankaya M, Halici MB, Coban TA (2018) A novel therapeutics agent: antioxidant effects of hydroxylfasudil on rat kidney and liver tissues in a protamine sulphate-induced cystitis rat model; preliminary results. Artif Cells Nanomed Biotechnol 46(sup2):9–14. https://doi.org/10.1080/21691401.2018.1449120
Simioni C, Zauli G, Martelli AM, Vitale M, Sacchetti G, Gonelli A, Neri LM (2018) Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 9(24):17181–17198. https://doi.org/10.18632/oncotarget.24729
Budak H, Ceylan H, Kocpinar EF, Gonul N, Erdogan O (2014) Expression of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in oxidative stress induced by long-term iron toxicity in rat liver. J Biochem Mol Toxicol 28(5):217–223. https://doi.org/10.1002/jbt.21556
Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sanchez-Perez P, Cadenas S, Lamas S (2015) Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 6:183–197. https://doi.org/10.1016/j.redox.2015.07.008
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13(3):3145–3175. https://doi.org/10.3390/ijms13033145
Pannala VR, Bazil JN, Camara AKS, Dash RK (2013) A biophysically based mathematical model for the catalytic mechanism of glutathione reductase. Free Radic Biol Med 65:1385–1397. https://doi.org/10.1016/j.freeradbiomed.2013.10.001
Prusty BK, Bohme L, Bergmann B, Siegl C, Krause E, Mehlitz A, Rudel T (2012) Imbalanced oxidative stress causes chlamydial persistence during non-productive human herpes virus co-infection. PLoS One 7(10):e47427. https://doi.org/10.1371/journal.pone.0047427
Funding
Atatürk University Scientific Research Projects Coordination Commission (ATAUNI-BAP) provided financial support for the present study with project number PRJ2010/277, PRJ2015/97, and FAD-2018-6352.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: HB (group leader) and OE. Performed the experiments: HB, EFK, NGB, HC, and SNK. Analyzed the data: HB, EFK, NGB, and SNK. Contributed reagents/materials/analysis tools: HB and OE. Wrote the paper: HB, EFK, NGB, and SNK. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Ethical approval for the present research was acquired from the Local Animal Care Committee of Atatürk University, Turkey.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kocpinar, E.F., Gonul Baltaci, N., Ceylan, H. et al. Effect of a Prolonged Dietary Iron Intake on the Gene Expression and Activity of the Testicular Antioxidant Defense System in Rats. Biol Trace Elem Res 195, 135–141 (2020). https://doi.org/10.1007/s12011-019-01817-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01817-0