Abstract
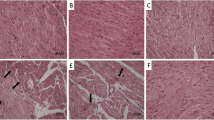
The anticancer drug cyclophosphamide (CP) has nephrotoxic effects besides its urotoxicity, which both in turn limit its clinical utility. The nephrotoxicity of CP is less common compared to its urotoxicity, and not much importance has been given for the study of mechanism of CP-induced nephrotoxicity so far. Overproduction of reactive oxygen species (ROS) during inflammation is one of the reasons of the kidney injury. Selenoproteins play crucial roles in regulating ROS and redox status in nearly all tissues; therefore, in this study, the nephrotoxicity of CP and the possible protective effects of seleno l-methionine (SLM) on rat kidneys were investigated. Forty-two Sprague–Dawley rats were equally divided into six groups of seven rats each. The control group received saline, and other rats were injected with CP (100 mg/kg), SLM (0.5 or 1 mg/kg), or CP + SLM intraperitoneally. Malondialdehyde (MDA) and glutathione (GSH) levels in kidney homogenates of rats were measured, and kidney tissues were examined under the microscope. CP-treated rats showed a depletion of renal GSH levels (28% of control), while CP + SLM-injected rats had GSH values close to the control group. MDA levels increased 36% of control following CP administration, which were significantly decreased after SLM treatment. Furthermore, these biochemical results were supported by microscopical observations. In conclusion, the present study not only points to the therapeutic potential of SLM in CP-induced kidney toxicity but also indicates a significant role for ROS and their relation to kidney dysfunction.



Similar content being viewed by others
References
Dollery C (1999) Cyclophosphamide. Therapeutic drugs. Churchill Livingstone, Edinburg, pp 349–353
Kopecna L (2001) Late effects of anticancer therapy on kidney function in children with acute lymphoblastic leukemia. Bratisl Lek Listy 102:357–360
Okamura T, Garland EM, Taylor RJ, Johansson SL, Cohen SM (1992) The effect of cyclophosphamide administration on the kidney of the rat. Toxicol Lett 63:261–276
Gustafsson LL, Eriksson L, Dahl S, Eleborg L (1996) Cyclophosphamide induced acute liver failure requiring transplantation in a patient with genetically deficient debrisoquine metabolism: a causal relationship? J Int Med 240:311–314
Rossi R, Godde A, Kleinebrand A, Riepenhausen M, Boos J, Ritter J (1994) Unilateral nephrectomy and cisplatin as risk factor of ifosfamide-induced nephrotoxicity: analysis of 120 patients. J Clin Oncol 12:159–165
Sugumar E, Kanakasabapathy I, Abraham P (2007) Normal plasma creatinine level despite histological evidence of damage and increased oxidative stress in the kidneys of cyclophosphamide treated rats. Clin Chim Acta 376:244–245
Abraham P, Rabi S (2009) Nitrosative stress, protein tyrosine nitration, PARP activation and NAD depletion in the kidneys of rats after single dose of cyclophosphamide. Clin Exp Nephrol 13:281–287. doi:10.1007/s10157-009-0160-z
Kern JC, Kehrer JP (2002) Acrolein-induced cell death: a caspase-influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact 139:79–95
Arumugam N, Sivakumar V, Thanislass J, Devaraj H (1997) Effects of acrolein on rat liver antioxidant defense system. Indian J Exp Biol 35:1373–1374
Mythili Y, Sudharsan PT, Selvakumar E, Varalakshmi P (2004) Protective effect of dl-a-lipoic acid on cyclophosphamide induced oxidative cardiac injury. Chem Biol Interact 151:13–19
Kawanishi M, Matsuda T, Nakayama A, Takebe H, Matsui S, Yagi T (1998) Molecular analysis of mutations induced by acrolein in human fibroblast cells using supf shuttle vector plasmids. Mut Res Gen Toxicol Environ Mutagen 417:65–73
Fouladi M, Stempak D, Gammon J (2001) Phase I trial of a twice-daily regimen of amifostine with ifosfamide, carboplatin and etoposide chemotherapy in children with refractory carcinoma. Cancer 92:914–923
Schrauzer GN (2003) The nutritional significance, metabolism, and toxicology of selenomethionine. Adv Food Nutr Res 47:73–112
Flohe L, Gunzler WA, Loschen G (1979) The glutathione peroxidase reaction: a key to understand the selenium requirement of mammals. Trace metals in health and disease. Raven, New York, pp 263–286
Bauer G, Wendel A (1980) The activity of the peroxide-metabolizing system in human colon carcinoma. J Cancer Res Clin Oncol 97:267–273
Cao S, Durrani FA, Rustum YM (2004) Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res 10:2561–2569
Ayhanci A, Uyar R, Aral E, Kabadere S, Appak S (2008) Protective effect of zinc on cyclophosphamide-induced hematoxicity and urotoxicity. Biol Trace Elem Res 126:186–193
Mohan IK, Khan M, Shobha JC, Naidu MU, Prayag A, Kuppusamy P (2006) Protection against cisplatin-induced nephrotoxicity by Spirulina in rats. Cancer Chemother Pharmacol 586:802–808
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Levine LA, Richie JP (1989) Urological complications of cyclophosphamide. J Urol 141:1063–1069
Ayhanci A, Yaman S, Sahinturk V, Uyar R, Bayramoglu G, Senturk H, Altuner Y, Appak S, Gunes S (2009) Protective effect of seleno-L-methionine on cyclophosphamide-induced urinary bladder toxicity in rats. Biol Trace Elem Res. doi:10.1007/s 12011-009-8458-y
Philips FS, Sternberg SS, Cronin AP, Vidal PM (1961) Cyclophosphamide and urinary bladder toxicity. Cancer Res 21:1577–1589
Levine S, Sowinski R (1974) Cyclophosphamide-induced cerebral and visceral lesions in rats. Arch Pathol 98:177–182
Hsu HC, Tsai HM (1982) Cyclophosphamide-induced glomerular injury in newborn mice. Lab Invest 47:281–285
Senthilkumar S, Devaki T, Manohar BM, Babu MS (2006) Effect of squalene on cyclophosphamide-induced toxicity. Clin Chim Acta 364:335–342
Cayir K, Karadeniz A, Yildirim A, Kalkan Y, Karakoc A, Keles M, Tekin SB (2009) Protective effect of L-carnitine against cisplatin-induced liver and kidney oxidant injury in rats. Cent Eur J Med 4(2):184–191
Lu Y, Cederbaum A (2007) The mode of cisplatin-induced cell death in CYP2E1-overexpressing HepG2 cells: modulation by ERK, ROS, glutathione, and thioredoxin. Free Radic Biol Med 43:1061–1075
Nannelli A, Messina A, Marini S, Trasciatti S, Longo V, Gervasi PG (2007) Effects of the anticancer dehydrotarplatin on cytochrome P450 and antioxidant enzymes in male rat tissues. Arch Toxicol 81:479–487
Whanger PD (1981) Selenium and heavy metal toxicity. In: Spallholz JE, Martin JL, Ganther HE (eds) Selenium in biology and medicine. AVI, Westport, pp 230–255
Diplock AT, Watkins WJ, Heurson M (1986) Selenium and heavy metals. Ann Clin Res 18:55–60
Ip C (1986) Selenium and experimental cancer. Ann Clin Res 18:22–29
Jamba L, Nehru B, Bansal MP (1997) Selenium supplementation during cadmium exposure: changes in antioxidant enzymes and the ultrastructure of the kidney. The Journal of Trace Elements in Experimental Medicine 10:233–242
Gray KJ, Engelmann UH, Fishman IJ (1986) Evaluation of misoprostol cytoprotection of the bladder with cyclophosphamide (cytoxan) therapy. J Urol 133:497–500
Furst A (2002) Can nutrition affect chemical toxicity? Int J Toxicol 21:419–424
Tripathi DN, Jena GB (2008) Ebselen attenuates cyclophosphamide-induced oxidative stress and DNA damage in mice. Free Radic Res 42(11):966–977
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayhanci, A., Günes, S., Sahinturk, V. et al. Seleno l-Methionine Acts on Cyclophosphamide-Induced Kidney Toxicity. Biol Trace Elem Res 136, 171–179 (2010). https://doi.org/10.1007/s12011-009-8535-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-009-8535-2