Abstract
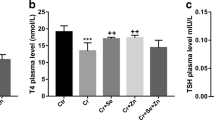
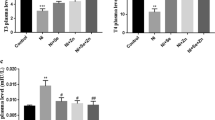
The aim of this study was to evaluate the potential benefit of combined treatment with zinc (Zn) and selenium (Se) in reversing cadmium (Cd)-induced thyroid dysfunction compared to Se or Zn treatment alone in rats exposed to Cd. For this purpose, 30 adult male Wistar albino rats were equally divided into control and four treated groups receiving either 200 ppm Cd (as CdCl2), 200 ppm Cd + 500 ppm Zn (as ZnCl2), 200 ppm Cd + 0.1 ppm Se (as Na2SeO3), or 200 ppm Cd + 500 ppm Zn + 0.1 ppm Se in their drinking water for 35 days. The results showed that Cd exposure increased significantly the relative thyroid weight (RTW), the thyroid Cd concentration, and the serum thyroid stimulating hormone (TSH) level, whereas the serum thyroxine (T4) level was decreased compared to control rats. The treatment of Cd-exposed rats with Se alone only partially protected from the Cd-induced decrease in serum T4 level. The treatment of Cd-exposed animals with Zn alone partially protected against Cd-induced thyroid dysfunction by maintaining normal RTW and by decreasing Cd concentration in the thyroid. It also partially prevents Cd-induced decrease in serum T4 level. The combined treatment of Cd-exposed animals with Se and Zn induced a more significant decrease in the thyroid Cd concentration than the Zn supplement and a total correction of the RTW. This treatment was also more effective than that with Se or Zn alone in reversing Cd-induced decrease in serum T4 level and Cd-induced increase in serum TSH level. Se and Zn can have a synergistic role against Cd-induced thyroid dysfunction.





Similar content being viewed by others
References
Nordberg G (1972) Cadmium metabolism and toxicity. Environ Physiol Biochem 2:7–36
World Health Organisation (1992) Environmental health criteria, 134 Cadmium. IPCS, Geneva
Yoshizuka M, Mori N, Hamasaki K, Tanaka I, Yokoyama M, Hara K (1991) Cadmium toxicity in the thyroid gland of pregnant rats. Exp Mol Pathol 55:97–104
Gupta P, Kar A (1999) Cadmium induced thyroid dysfunction in chicken: hepatic type I iodothyronine 5¢-monodeiodinase activity and role of lipid peroxidation. Comp Biochem Physiol C 123:39–44
Pilat-Marcinkiewicz B, Sawicki B, Brzoska MM, Jakoniuk JM (2002) Effect of chronic administration of cadmium on the rat thyroid: radioimmunological and immunohistochemical studies. Folia Histochem Cytobiol 40:189–90
Pilat-Marcinkiewicz B, Malgorzata M, Brzoska MM, Sawicki B, Jakoniuk JM (2003) Structure and function of thyroid follicular cells in female rats chronically exposed to cadmium. Bull Vet Inst PuLawy 47:157–163
Dickson RC, Tomlinson RH (1967) Selenium in blood and human tissues. Clin Chim Acta 16:311–321
Beckett GJ, Arthur JR (2005) Selenium and endocrine systems. J Endocrinol 184:455–465
Vrca VB, Skerb F, Cepelak I, Romic Z, Mayer L (2004) Supplementation with antioxidants in the treatment of Graves’ disease; the effect on glutathione peroxidase activity and concentration of selenium. Clin Chim Acta 341:55–63
Pekary AE, Lukaski HC, Mena I, Hershman JM (1991) Processing of TRH precursor peptides in rat brain and pituitary is zinc dependent. Peptides 12:1025–1032
Chen MD Lin PY, Lin WH (1998) Zinc supplementation on serum levels and hepatic conversion of thyroid hormones in obese (ob/ob) mice. Biol Trace Elem Res 61:89–96
Freake HC, Govoni KE, Guda K, Huang C, Zinn SA (2001) Actions and interactions of thyroid hormone and zinc status in growing rats. J Nutr 131:1135–1141
Baltaci AK, Mgulkoc R, Bediz CS, Ugur A (2003) Pinealectomy and zinc deficiency have opposite effects on thyroid hormones in rats. Endocr Res 29:473–481
Baltaci AK, Mogulko R, Kul A, Bediz CS, Ugur A (2004) Opposite effects of zinc and melatonin on thyroid hormones in rats. Toxicology 195:69–75
Brzóska MM, Moniuszko-Jakoniuk J (2000) Interactions between cadmium and zinc in the organism. Food Chem Toxicol 39:967–980
Lamphere DN, Dorn CR, Reddy CS, Meyer AW (1984) Reduced cadmium body burden in cadmium-exposed calves fed supplemental zinc. Environ Res 33:119–129
Chowdhury BA, Friel JK, Chandra RK (1987) Cadmium-induced immunopathology is prevented by zinc administration in mice. J Nutr 117:1788–1794
Jacquillet G, Barbier O, Cougnon M, Tauc M, Namorado MC, Martin D, Reyes JL, Poujeol P (2006) Zinc protects renal function during cadmium intoxication in the rat. Am J Physiol Renal Physiol 290:127–137
Brzóska MM, Galażyn-Sidorczuk M, Rogalska J, Roszczenko A, Jurczuk M, Majewska K, Moniuszko-Jakoniuk J (2008) Beneficial effect of zinc supplementation on biomechanical properties of femoral distal end and femoral diaphysis of male rats chronically exposed to cadmium. Chem Biol Interact 171:312–324
Jemai H, Messaoudi I, Chaouch A, Kerkeni A (2007) Protective effect of zinc supplementation on blood antioxidant defense system in rats exposed to cadmium. J Trace Elem Med Biol 21:269–273
Sugawara N, Sugawara C (1984) Selenium protection against testicular lipid peroxidation from cadmium. J Appl Biochem 6:199–204
Jamba L, Nehru B, Bansal MP (2000) Effect of selenium supplementation on the influence of cadmium on glutathione and glutathione peroxidase system in mouse liver. J Trace Elem Exp Med 13:299–304
El-Sharaky AS, Newairy AA, Badreldeen MM, Eweda SM, Sheweita SA (2007) Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 235:185–193
Newairy AA, El-Sharaky AS, Badreldeen MM, Eweda SM, Sheweita SA (2007) The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology 242:23–30
Stajn A, Zikic RV, Ognjanovic B, Saicic ZS, Pavlovic SZ, Kostic MM, Petrovic VM (1997) Effect of cadmium and selenium on the antioxidant defense system in rat kidneys. Comp Biochem Physiol 117:167–172
Brzóska MM, Moniuszko-Jakoniuk J (2005) Bone metabolism of male rats chronically exposed to cadmium. Toxicol Appl Pharmacol 207:195–211
Khotimchenko M, Segushchenko I, Khotim chenko Y (2004) The effects of low-esterified pectin on lead-induced thyroid injury in rats. Environ Toxicol Pharmacol 17:67–71
Prakash P, Kumar PG, Lalorya M, Javeri T, Parihar MS (1997) Superoxide anion radical production as a cadmium-mediated mechanism of toxicity in avian thyroid: an electron spin resonance study by spin trapping. Comp Biochem Physiol C 118:89–95
Gupta A, Kar A (1997) Role of testosterone in ameliorating the cadmium induced inhibition of thyroid function in adult male mouse. Bull Environ Contam Toxicol 58:422–428
Shupnik MA, Ardisson LJ, Meskell MJ, Bornstein J, Ridgway EC (1986) Triiodothyronine (T3) regulation of thyrotropin subunit gene transcription is proportional to T3 nuclear receptor occupancy. Endocrinology 118:367–371
Badiello R, Feroci G, Fini A (1996) Interaction between trace elements: selenium and cadmium ions. J Trace Elem Med Biol 10:156–162
Rana SV, Verma S (1996) Protective effects of GSH, vitamin E and selenium on lipid peroxidation in cadmium-fed rats. Biol Trace Elem Res 51:161–168
He R, Bao KG (1994) Effects of selenium and DTPA on cadmium excretion in rats. Chung Hua Yu Fang I Hsueh Tsa Chih 28:97–99
Meyer SA, House WA, Welch RM (1982) Some metabolic interrelationships between toxic levels of cadmium and nontoxic levels of selenium fed to rats. J Nutr 112:954–961
Oliver JW, Sachan DS, Su P, Applehans FM (1987) Effects of zinc deficiency on thyroid function. Drug Nutr Interact 5:113–124
Leblondel G, Allin P (1989) Effects of thyroparathyroidectomy and thyroxin and calcitonin on the tissue distribution of twelve elements in rats. Biol Trace Elem Res 9:171–183
Leblondel G, Le Bouil A, Allin P (1992) Influence of thyroparathyroidectomy and thyroxin replacement on Cu and Zn cellular distribution and on metallothionein level and induction in rats. Biol Trace Elem Res 32:281–288
Baltaci AK, Mogulkoc R, Bediz CS, Kutlu S, Sandal S, Dogru O (1999) The effects of hypothyroidism on plasma levels of zinc in rats. J Turkey Med 6:105–109
Bray TM, Bettger WJ (1990) The physiological role of zinc as an antioxidant. Free Radic Biol Med 8:281–291
Girotti AW, Thomas JP, Jordan JE (1985) Inhibitory effect of zinc (II) on free radical lipid peroxidation in erythrocyte membranes. Free Radic Biol Med 1:395–401
Sato M, Bremner I (1993) Oxygen free radicals and metallothionein. Free Radic Biol Med 14:325–337
Napolitano G, Pakla G, Lio S, Bucci I, De Remigis P, Stuppia L, Monaco F (1990) Is zinc deficiency a cause of subclinical hypothyroidism in Down syndrome. Ann Genet 33:9–15
Xiao P, Jia XD, Zhong WJ, Jin XP, Nordberg G (2002) Restorative effects of zinc and selenium on cadmium-induced kidney oxidative damage in rats. Biomed Environ Sci 5:67–74
He PP, Lv XZ, Wang GY (2004) Effects of Se and Zn supplementation on the antagonism against Pb and Cd in vegetables. Environ Int 30:167–172
Feroci G, Badiello R, Fini A (2005) Interactions between different selenium compounds and zinc, cadmium and mercury. J Trace Elem Med Biol 18:227–234
Author information
Authors and Affiliations
Corresponding author
Additional information
Hammouda and Messaoudi have contributed equally.
Rights and permissions
About this article
Cite this article
Hammouda, F., Messaoudi, I., El Hani, J. et al. Reversal of Cadmium-Induced Thyroid Dysfunction by Selenium, Zinc, or Their Combination in Rat. Biol Trace Elem Res 126, 194–203 (2008). https://doi.org/10.1007/s12011-008-8194-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-008-8194-8