Abstract
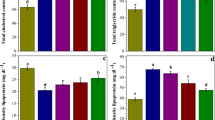
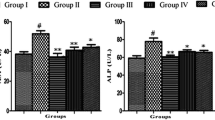
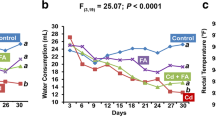
In the present work, protective effect of tetrahydrocurcumin (THC) against oxidative damages in cadmium (Cd)-induced toxicity in rats was evaluated. Cd is an important environmental and industrial toxicant that affects almost all the organs, especially liver. Liver is the major organ responsible for the metabolism and the primary target for many toxic chemicals and drugs. Effect of THC, the curcumin-derived polyphenolic compound on Cd-induced oxidative stress and hepatic damage was evaluated using male albino Wistar rats. In Cd-administered rats (5 mg/kg body weight (b.w.), orally for 4 weeks), activities of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH) and gamma glutamyl transferase (GGT) were significantly increased in serum with the elevated level of bilirubin. Red blood cells (RBC), haemoglobin contents and haematocrit values were also significantly decreased in Cd-treated rats. In addition, the levels of lipid peroxidation markers like thiobarbituric acid reactive substances (TBARS), lipid hydroperoxides (LHP), protein carbonyl contents (PCC) and conjugated dienes (CD) were significantly increased followed by the significant decrease in the levels of reduced glutathione (GSH), total sulphydryl groups (TSH), ascorbic acid (vitamin C) and vitamin E in liver of Cd-administered rats. Oral administration of THC (20, 40 and 80 mg/kg b.w.) followed by Cd for 4 weeks showed a significant restoration of the above changes to near normal. Histopathological changes observed in Cd intoxicated hepatic tissues were minimized on treatment with THC. This study suggests that THC at the dose of 80 mg/kg b.w. effectively subdues the Cd-induced toxicity and controls the free radical-induced liver damage in rats.





Similar content being viewed by others
References
Xiao, P., Jia, X. D., Zhong, W. J., Jin, X. P., & Nordberg, G. U. N. N. A. R. (2002). Restorative effects of zinc and selenium on cadmium-induced kidney oxidative damage in rats. Biomedical and Environmental Science, 15(1), 67–74.
Bernard, A. (2008). Cadmium and its adverse effects on human health. Indian Journal of Medical Research, 128, 557–564.
Thijssen, S., Cuypers, A., Maringwa, J., Smeets, K., Horemans, N., Lambrichts, I., & Van Kerkhove, E. (2007). Low cadmium exposure triggers a biphasic oxidative stress response in mice kidneys. Toxicology, 236(1), 29–41.
Kara, H., Cevik, A., Konar, V., Dayangac, A., & Yilmaz, M. (2007). Protective effects of antioxidants against cadmium-induced oxidative damage in rat testes. Biological Trace Element Research, 120(1–3), 205–211.
Konar, V., Kara, H., Yilmaz, M., Dayangac, A., & Karatas, F. (2007). Effects of selenium and vitamin E, in addition to melatonin, against oxidative stress caused by cadmium in rats. Biological Trace Element Research, 118(2), 131–137.
Everett, C. J., & Frithsen, I. L. (2008). Association of urinary cadmium and myocardial infarction. Environmental Research, 106(2), 284–286.
Yiin, S. J., Chern, C. L., Sheu, J. Y., Tseng, W. C., & Lin, T. H. (1999). Cadmium-induced renal lipid peroxidation in rats and protection by selenium. Journal of Toxicology and Environmental Health, A, 57(6), 403–413.
Pandey, S., Parvez, S., Ansari, R. A., Ali, M., Kaur, M., Hayat, F., et al. (2008). Effects of exposure to multiple trace metals on biochemical, histological and ultrastructural features of gills of a freshwater fish, Channa punctata Bloch. Chemico-Biological Interactions, 174(3), 183–192.
Nawrot, T., Plusquin, M., Hogervorst, J., Roels, H. A., Celis, H., Thijs, L., et al. (2006). Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncology, 7(2), 119–126.
Eybl, V., Kotyzová, D., & Bludovská, M. (2004). The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicology Letters, 151(1), 79–85.
Prakash, A. S., Tran, H. P., Peng, C., Koyalamudi, S. R., & Dameron, C. T. (2000). Kinetics of DNA alkylation, depurination and hydrolysis of anti diol epoxide of benzo (a) pyrene and the effect of cadmium on DNA alkylation. Chemico-Biological Interactions, 125(2), 133–150.
Youdim, K. A., & Joseph, J. A. (2001). A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radical Biology and Medicine, 30(6), 583–594.
Shagirtha, K., & Pari, L. (2011). Hesperetin, a citrus flavonone, attenuates cadmium-induced nephrotoxicity in rat. Biomedicine and Preventive Nutrition, 1(2), 139–145.
Anand, P., Kunnumakkara, A. B., Newman, R. A., & Aggarwal, B. B. (2007). Bioavailability of curcumin: problems and promises. Molecular Pharmaceutics, 4(6), 807–818.
Majeed, M., Badmaev, V., Uma, S., & Rajenderan, J. R. (1995). Curcuminoids: antioxidant Phytonutrients (pp. 1–24). New Jersey: Nutreiscience publishers.
Sugiyama, Y., Kawakishi, S., & Osawa, T. (1996). Involvement of the β-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochemical Pharmacology, 52(4), 519–525.
Lai, C. S., Wu, J. C., Yu, S. F., Badmaev, V., Nagabhushanam, K., Ho, C. T., & Pan, M. H. (2011). Tetrahydrocurcumin is more effective than curcumin in preventing azoxymethane-induced colon carcinogenesis. Molecular Nutrition & Food Research, 55(12), 1819–1828.
Lin, J. K., & Lin-Shiau, S. Y. (2001). Mechanisms of cancer chemoprevention by curcumin. Pro. Nat. Sci. Coun. Rep China Part B, Life Sciences, 25(2), 59–66.
Naito, M., Wu, X., Nomura, H., Kodama, M., Kato, Y., Kato, Y., & Osawa, T. (2002). The protective effects of tetrahydrocurcumin on oxidative stress in cholesterol-fed rabbits. Journal of Atherosclerosis and Thrombosis, 9(5), 243–250.
Pari, L., & Murugan, P. (2004). Protective role of tetrahydrocurcumin against erythromycin estolate-induced hepatotoxicity. Pharmaceutical Research, 49(5), 481–486.
Renugadevi, J., & Prabu, S. M. (2009). Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology, 256(1), 128–134.
Amudha, K., & Pari, L. (2011). Beneficial role of naringin, a flavanoid on nickel induced nephrotoxicity in rats. Chemico-Biological Interactions, 193(1), 57–64.
Shirley, R. L., Benne, E. J., & Miller, E. J. (1949). Cadmium in biological materials and foods. Analytical Chemistry, 21(2), 300–303.
Malloy, H. T., & Evelyn, K. A. (1937). The determination of bilirubin with the photoelectric colorimeter. The Journal of Biological Chemistry, 119(2), 481–490.
Niehaus, W. G., & Samuelsson, B. (1968). Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. European Journal of Biochemistry, 6(1), 126–130.
Jiang, Z. Y., Hunt, J. V., & Wolff, S. P. (1992). Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Analytical Chemistry, 202(2), 384–389.
Levine, R. L., Garland, D., Oliver, C. N., Amici, A., Climent, I., Lenz, A.-G., Ahn, B.-W., Shaltiel, S., & Stadtman, E. R. (1990). Determination of carbonyl content in oxidatively modified proteins. Methods Enzymology, 186, 465–478.
Rao, K. S., & Recknagel, R. O. (1968). Early onset of lipoperoxidation in rat liver after carbon tetrachloride administration. Experimental and Molecular Pathology, 9(2), 271–278.
Kakkar, P., Das, B., & Viswanathan, P. N. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry and Biophysics, 21(2), 130–132.
Sinha, A. K. (1972). Colorimetric assay of catalase. Analytical Chemistry, 47(2), 389–394.
Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G., & Hoekstra, W. (1973). Selenium: biochemical role as a component of glutathione peroxidase. Science, 179(4073), 588–590.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases the first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249(22), 7130–7139.
Horn, H. D., & Burns, F. H. (1978). Assay of glutathione reductase activity. In H. V. Bergemeyer (Ed.), Methods of enzymatic analysis (pp. 142–146). New York: Academic Press.
Beutler, E. (1983). Active transport of glutathione disulfide from erythrocytes. In A. Larson, S. Orrenius, A. Holmgren, & B. Mannerwik (Eds.), Functions of glutathione-biochemical, physiological (pp. 65–74). New York: Toxicological and Clinical Aspects. Raven Press.
Omaye, S. T., Turnbull, J. D., & Sauberlich, H. E. (1979). Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods in enzymology, 62, 3–11.
Desai, I. D. (1984). Vitamin E analysis methods for animal tissues [Alpha tocopherol]. Methods in Enzymology (USA).
Zahler, W. L., & Cleland, W. W. (1968). A specific and sensitive assay for disulfides. Journal of Biological Chemistry, 243(4), 716–719.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254.
Duncan, D. B. (1957). Multiple range tests for correlated and heteroscedastic means. Biometrics, 13(2), 164–176.
Pari, L., & Murugavel, P. (2005). Role of diallyl tetrasulfide in ameliorating the cadmium induced biochemical changes in rats. Environmental Toxicology and Pharmacology, 20(3), 493–500.
Girault, L., Boudou, A., & Dufourc, E. J. (1998). 113 Cd-, 31 P-NMR and fluorescence polarization studies of cadmium (II) interactions with phospholipids in model membranes. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1414(1), 140–154.
Sundberg, A., Appelkvist, E. L., Dallner, G., & Nilsson, R. (1994). Glutathione transferases in the urine: sensitive methods for detection of kidney damage induced by nephrotoxic agents in humans. Environmental Health Perspectives, 102(Suppl 3), 293.
Drozdz, R., Parmentier, C., Hachad, H., Leroy, P., Gérard Siest, G., & Wellman, M. (1998). γ-glutamyltransferase dependent generation of reactive oxygen species from a glutathione/transferrin system. Free Radical Biology and Medicine, 25(7), 786–792.
Whitfield, J. B. (2001). Gamma glutamyl transferase. Critical Reviews in Clinical Laboratory Sciences, 38(4), 263–355.
Lee, D. H., Blomhoff, R., & Jacobs, D. R. (2004). Review is serum gamma glutamyltransferase a marker of oxidative stress? Free Radical Research, 38(6), 535–539.
Sarkar, S., Yadav, P., & Bhatnagar, D. (1998). Lipid peroxidative damage on cadmium exposure and alterations in antioxidantsystem in rat erythrocytes: a study with relation to time. Biometals, 11(2), 153–157.
Luchese, C., Zeni, G., Rocha, J. B., Nogueira, C. W., & Santos, F. W. (2007). Cadmium inhibits δ-aminolevulinate dehydratase from rat lung in vitro: interaction with chelating and antioxidant agents. Chemico-Biological Interactions, 165(2), 127–137.
Hamada, T., Tanimoto, A., Arima, N., Ide, Y., Sasaguri, T., Shimajiri, S., & Sasaguri, Y. (1998). Altered membrane skeleton of red blood cells participates in cadmium-induced anemia. IUBMB Life, 45(4), 841–847.
Kłapcińska, B., Poprzecki, S., Dolezych, B., & Kimsa, E. (2000). Cadmium-induced changes in hematology and 2,3-DPG levels in rats. Bulletin of Environmental Contamination and Toxicology, 64(1), 93–99.
Klaassen, C. D., Liu, J., & Choudhuri, S. (1999). Metallothionein: an intracellular protein to protect against cadmium toxicity. Annual Review of Pharmacology and Toxicology, 39(1), 267–294.
Nakamoto, K. (1977). Infrared and Raman spectra of inorganic and coordination compounds. Wiley.
Hussain, T., Shukla, G. S., & Chandra, S. V. (1987). Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies. Pharmacology & Toxicology, 60(5), 355–358.
Manca, D., Ricard, A. C., Trottier, B., & Chevalier, G. (1991). Studies on lipid peroxidation in rat tissues following administration of low and moderate doses of cadmium chloride. Toxicology, 67(3), 303–323.
Stohs, S. J., & Bagchi, D. (1995). Oxidative mechanisms in the toxicity of metal ions. Free Radical Research, 18(2), 321–336.
Rikans, L. E., & Yamano, T. (2000). Mechanisms of cadmium-mediated acute hepatotoxicity. Journal of Biochemical and Molecular Toxicology, 14(2), 110–117.
Wang, Y., Fang, J., Leonard, S. S., & Rao, K. M. K. (2004). Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Journal of Biochemical and Molecular Toxicology, 36(11), 1434–1443.
Ognjanović, B. I., Pavlović, S. Z., Maletić, S. D., Zikić, R. V., Stajn, A. S., Radojicić, R. M., et al. (2003). Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiological Research, 52(5), 563–570.
Prabu, M. S., Selvarajan, N., Hemalatha, S., & Rameshkumar, T. (2008). Hepatoprotective effect of Andrographis paniculata against cadmium induced toxicity in male Wistar rats. Toxicology Int., 15(1), 21.
Borsook, H., & Keighley, G. (1933). Oxidation-reduction potential of ascorbic acid (vitamin C). Proceedings of the National Academy of Sciences, 19(9), 875–878.
Chatterjee, G. C., Banerjee, S. K., & Pal, D. R. (1972). Cadmium administration and L-ascorbic acid metabolism in rats: effect of L-ascorbic acid supplementation. Internationale Zeitschrift fur vitamin-und Ernahrungsforschung International Journal for Vitamin and Nutrition Research, 43(3), 370–377.
Hudecova, A., & Ginter, E. (1992). The influence of ascorbic acid on lipid peroxidation in Guinea pigs intoxicated with cadmium. Food and Chemical Toxicology, 30(12), 1011–1013.
Pavlovic, S. Z., Ognjanovic, B. I., Stajn, A. S., Zikic, R. V., Saicic, Z. S., & Petrovic, V. M. (2001). The effect of coenzyme Q 10 on blood ascorbic acid, vitamin E, and lipid peroxide in chronic cadmium intoxication. Journal of Environmental Pathology, Toxicology and Oncology, 20(2).
Rana, S. V. S., & Verma, S. (1996). Protective effects of GSH, vitamin E, and selenium on lipid peroxidation in cadmium-fed rats. Biological Trace Element Research, 51(2), 161–168.
Griffith, O. W. (1999). Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radical Biology and Medicine, 27(9), 922–935.
Navarro, F., Arroyo, A., Martín, S. F., Bello, R. I., De Cabo, R., Burgess, J. R., et al. (1999). Protective role of ubiquinone in vitamin E and selenium-deficient plasma membranes. BioFactors, 9(2–4), 163–170.
El-Demerdash, F. M., Yousef, M. I., Kedwany, F. S., & Baghdadi, H. H. (2004). Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β-carotene. Food and Chemmical Toxicology, (10), 1563–1571.
Bauer, R., Demeter, I., Hasemann, V., & Johansen, J. T. (1980). Structural properties of the zinc site in Cu, Zn-superoxide dismutase; perturbed angular correlation of gamma ray spectroscopy on the Cu, 111 Cd-superoxide dismutase derivative. Biochemical and Biophysical Research Communications, 94(4), 1296–1302.
Li, W. D., Kagan, H. M., & Chou, I. N. (1994). Alterations in cytoskeletal organization and homeostasis of cellular thiols in cadmium-resistant cellsToxicology and. Applied Pharmacology, 126(1), 114–123.
Freeman, B. A., & Crapo, J. D. (1982). Biology of disease: free radicals and tissue injury. Laboratory investigation; a journal of technical methods and pathology, 47(5), 412–426.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82(1), 70–77.
Acknowledgements
The authors are deeply grateful to Ministry of Environment and Forests for the constant financial (19-35/2009-RE/09-11-2010) and moral support towards the successful completion of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramakrishnan, R., Elangovan, P. & Pari, L. Protective Role of Tetrahydrocurcumin: an Active Polyphenolic Curcuminoid on Cadmium-InducedOxidative Damage in Rats. Appl Biochem Biotechnol 183, 51–69 (2017). https://doi.org/10.1007/s12010-017-2430-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2430-7