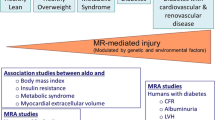
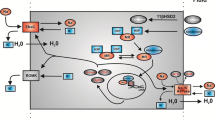
Abstract
In the past decades, we have extended the view of aldosterone effects beyond epithelial tissues. New evidence regarding the aldosterone/mineralocorticoid receptor (MR) pathway in active metabolic tissues, including adipose tissue, has confirmed its pathogenic role in systemic inflammation, endothelial dysfunction, insulin resistance, and dyslipidemia. Obesity, a current epidemic worldwide, increases aldosterone production by several adipocyte factors such as leptin but is also associated with local aldosterone production. In addition, obesity can modulate MR activation leading to signaling dysregulation and a pro-inflammatory profile of adipocytes. Current knowledge have deciphered that this phenotypical differences of obesity may be explained, at least in part, by novel non-genomic activation of MR, new inducers of aldosterone synthesis, and probably by several epigenetic modifications. In addition, with the understanding of the complex interplay of obesity, hormones, and receptors, targeted pharmacological therapy is expected and is currently under active research.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104(4):545–56.
Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84(2):489–539. doi:10.1152/physrev.00030.2003.
Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol. 2014;4(3):965–94. doi:10.1002/cphy.c130044.
Muller-Fielitz H, Lau M, Johren O, Stellmacher F, Schwaninger M, Raasch W. Blood pressure response to angiotensin II is enhanced in obese Zucker rats and is attributed to an aldosterone-dependent mechanism. Br J Pharmacol. 2012;166(8):2417–29. doi:10.1111/j.1476-5381.2012.01953.x.
Tomaschitz A, Pilz S, Ritz E, Obermayer-Pietsch B, Pieber TR. Aldosterone and arterial hypertension. Nat Rev Endocrinol. 2010;6(2):83–93. doi:10.1038/nrendo.2009.263.
Marver D, Kokko JP. Renal target sites and the mechanism of action of aldosterone. Miner Electrolyte Metab. 1983;9(1):1–18.
Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocorticoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96(6):643–50. doi:10.1161/01.RES.0000159937.05502.d1.
NRN Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–3.
Fuller PJ, Young MJ. Mechanisms of mineralocorticoid action. Hypertension. 2005;46(6):1227–35. doi:10.1161/01.HYP.0000193502.77417.17.
Naray-Fejes-Toth A, Fejes-Toth G. The sgk, an aldosterone-induced gene in mineralocorticoid target cells, regulates the epithelial sodium channel. Kidney Int. 2000;57(4):1290–4. doi:10.1046/j.1523-1755.2000.00964.x.
Lombes M, Alfaidy N, Eugene E, Lessana A, Farman N, Bonvalet JP. Prerequisite for cardiac aldosterone action. Mineralocorticoid receptor and 11 beta-hydroxysteroid dehydrogenase in the human heart. Circulation. 1995;92(2):175–82.
Takeda Y, Miyamori I, Inaba S, Furukawa K, Hatakeyama H, Yoneda T, et al. Vascular aldosterone in genetically hypertensive rats. Hypertension. 1997;29(1 Pt 1):45–8.
Zhou MY, Gomez-Sanchez CE, Gomez-Sanchez EP. An alternatively spliced rat mineralocorticoid receptor mRNA causing truncation of the steroid binding domain. Mol Cell Endocrinol. 2000;159(1–2):125–31.
Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54(3):537–43. doi:10.1161/HYPERTENSIONAHA.109.131110.
Rickard AJ, Young MJ. Corticosteroid receptors, macrophages and cardiovascular disease. J Mol Endocrinol. 2009;42(6):449–59. doi:10.1677/JME-08-0144.
Carvajal CA, Herrada AA, Castillo CR, Contreras FJ, Stehr CB, Mosso LM, et al. Primary aldosteronism can alter peripheral levels of transforming growth factor beta and tumor necrosis factor alpha. J Endocrinol Investig. 2009;32(9):759–65. doi:10.3275/6429.
Herrada AA, Contreras FJ, Marini NP, Amador CA, Gonzalez PA, Cortes CM, et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol. 2010;184(1):191–202. doi:10.4049/jimmunol.0802886.
Calhoun DA, Sharma K. The role of aldosteronism in causing obesity-related cardiovascular risk. Cardiol Clin. 2010;28(3):517–27. doi:10.1016/j.ccl.2010.04.001.
Pimenta E, Calhoun DA. Aldosterone and metabolic dysfunction: an unresolved issue. Hypertension. 2009;53(4):585–6. doi:10.1161/HYPERTENSIONAHA.108.123406.
Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150(11):776–83.
Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. doi:10.1210/er.2008-0024.
Vaidya A, Underwood PC, Hopkins PN, Jeunemaitre X, Ferri C, Williams GH, et al. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension. 2013;61(4):886–93. doi:10.1161/HYPERTENSIONAHA.111.00662. This report presents new information, whether novel indices of aldosterone responses to dietary sodium modulation, could predict cardiometabolic risk factors. The integration of aldosterone suppression and stimulation would provide an improved representation of aldosterone physiology in disease states that could offer new insights in the pathogenesis and treatment of cardiometabolic derangements.
Harada E, Mizuno Y, Katoh D, Kashiwagi Y, Morita S, Nakayama Y, et al. Increased urinary aldosterone excretion is associated with subcutaneous not visceral, adipose tissue area in obese individuals: a possible manifestation of dysfunctional subcutaneous adipose tissue. Clin Endocrinol. 2013;79(4):510–6. doi:10.1111/cen.12083.
Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91(2):454–9. doi:10.1210/jc.2005-1733.
Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, et al. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation. 2007;116(9):984–92. doi:10.1161/CIRCULATIONAHA.107.708537.
Rowe JW, Tobin JD, Rosa RM, Andres R. Effect of experimental potassium deficiency on glucose and insulin metabolism. Metab Clin Exp. 1980;29(6):498–502.
Ferrannini E, Galvan AQ, Santoro D, Natali A. Potassium as a link between insulin and the renin-angiotensin-aldosterone system. J Hypertens Suppl. 1992;10(1):S5–10.
Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids. 2014;91:54–60. doi:10.1016/j.steroids.2014.08.016.
Luther JM, Brown NJ. The renin-angiotensin-aldosterone system and glucose homeostasis. Trends Pharmacol Sci. 2011;32(12):734–9. doi:10.1016/j.tips.2011.07.006.
Luther JM, Luo P, Kreger MT, Brissova M, Dai C, Whitfield TT, et al. Aldosterone decreases glucose-stimulated insulin secretion in vivo in mice and in murine islets. Diabetologia. 2011;54(8):2152–63. doi:10.1007/s00125-011-2158-9.
Mosso LM, Carvajal CA, Maiz A, Ortiz EH, Castillo CR, Artigas RA, et al. A possible association between primary aldosteronism and a lower beta-cell function. J Hypertens. 2007;25(10):2125–30. doi:10.1097/HJH.0b013e3282861fa4.
Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab. 2010;95(4):1986–90. doi:10.1210/jc.2009-2521.
Williams TA, Monticone S, Urbanet R, Bertello C, Giraudo G, Vettor R, et al. Genes implicated in insulin resistance are down-regulated in primary aldosteronism patients. Mol Cell Endocrinol. 2012;355(1):162–8. doi:10.1016/j.mce.2012.02.007.
Rossi GP, Belfiore A, Bernini G, Fabris B, Caridi G, Ferri C, et al. Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93(7):2566–71. doi:10.1210/jc.2008-0251.
Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45(3):356–62. doi:10.1161/01.HYP.0000154361.47683.d3.
Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59(5):1069–78. doi:10.1161/HYPERTENSIONAHA.111.190223. Adipocytes possess aldosterone synthase and produce aldosterone in an Ang II/Ang II type 1 receptor/calcineurin/nuclear factor of activated T-cells–dependent manner. identifying adipocytes as a putative link between aldosterone and vascular dysfunction in diabetes mellitus–associated obesity.
Schinner S, Willenberg HS, Krause D, Schott M, Lamounier-Zepter V, Krug AW, et al. Adipocyte-derived products induce the transcription of the StAR promoter and stimulate aldosterone and cortisol secretion from adrenocortical cells through the Wnt-signaling pathway. Int J Obes. 2007;31(5):864–70. doi:10.1038/sj.ijo.0803508.
Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, et al. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17(12):3438–46. doi:10.1681/ASN.2006080944.
Lamounier-Zepter V, Ehrhart-Bornstein M, Bornstein SR. Mineralocorticoid-stimulating activity of adipose tissue. Best Pract Res Clin Endocrinol Metab. 2005;19(4):567–75. doi:10.1016/j.beem.2005.07.002.
Ehrhart-Bornstein M, Arakelyan K, Krug AW, Scherbaum WA, Bornstein SR. Fat cells may be the obesity-hypertension link: human adipogenic factors stimulate aldosterone secretion from adrenocortical cells. Endocr Res. 2004;30(4):865–70.
Goodfriend TL, Ball DL, Raff H, Bruder ED, Gardner HW, Spiteller G. Oxidized products of linoleic acid stimulate adrenal steroidogenesis. Endocr Res. 2002;28(4):325–30.
de Haro MC, Figueiredo VN, de Faria AP, Barbaro NR, Sabbatini AR, Quinaglia T, et al. High-circulating leptin levels are associated with increased blood pressure in uncontrolled resistant hypertension. J Hum Hypertens. 2013;27(4):225–30. doi:10.1038/jhh.2012.29.
Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, et al. The adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation. 2015. doi:10.1161/CIRCULATIONAHA.115.018226. This report presents key information on the adipokine leptin is a direct regulator of aldosterone synthase (CYP11B2) expression and aldosterone release and promotes cardiovascular dysfunction via aldosterone-dependent mechanisms.
Luo P, Dematteo A, Wang Z, Zhu L, Wang A, Kim HS, et al. Aldosterone deficiency prevents high-fat-feeding-induced hyperglycaemia and adipocyte dysfunction in mice. Diabetologia. 2013;56(4):901–10. doi:10.1007/s00125-012-2814-8.
Baudrand R, Pojoga LH, Romero JR, Williams GH. Aldosterone’s mechanism of action: roles of lysine-specific demethylase 1, caveolin and striatin. Curr Opin Nephrol Hypertens. 2014;23(1):32–7. doi:10.1097/01.mnh.0000436543.48391.e0.
Shibata S, Fujita T. The kidneys and aldosterone/mineralocorticoid receptor system in salt-sensitive hypertension. Curr Hypertens Rep. 2011;13(2):109–15. doi:10.1007/s11906-010-0175-6.
Zennaro MC, Le Menuet D, Viengchareun S, Walker F, Ricquier D, Lombes M. Hibernoma development in transgenic mice identifies brown adipose tissue as a novel target of aldosterone action. J Clin Invest. 1998;101(6):1254–60. doi:10.1172/JCI1915.
Viengchareun S, Penfornis P, Zennaro MC, Lombes M. Mineralocorticoid and glucocorticoid receptors inhibit UCP expression and function in brown adipocytes. Am J Physiol Endocrinol Metab. 2001;280(4):E640–9.
Penfornis P, Viengchareun S, Le Menuet D, Cluzeaud F, Zennaro MC, Lombes M. The mineralocorticoid receptor mediates aldosterone-induced differentiation of T37i cells into brown adipocytes. Am J Physiol Endocrinol Metab. 2000;279(2):E386–94.
Caprio M, Feve B, Claes A, Viengchareun S, Lombes M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007;21(9):2185–94. doi:10.1096/fj.06-7970com.
Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92(3):1023–33. doi:10.1210/jc.2006-1055.
Bluher M. Are there still healthy obese patients? Curr Opin Endocrinol Diabetes Obes. 2012;19(5):341–6. doi:10.1097/MED.0b013e328357f0a3.
Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299(3):E506–15. doi:10.1152/ajpendo.00586.2009.
Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117(17):2253–61. doi:10.1161/CIRCULATIONAHA.107.748640.
Hirata A, Maeda N, Nakatsuji H, Hiuge-Shimizu A, Okada T, Funahashi T, et al. Contribution of glucocorticoid-mineralocorticoid receptor pathway on the obesity-related adipocyte dysfunction. Biochem Biophys Res Commun. 2012;419(2):182–7. doi:10.1016/j.bbrc.2012.01.139.
Boscaro M, Giacchetti G, Ronconi V. Visceral adipose tissue: emerging role of gluco- and mineralocorticoid hormones in the setting of cardiometabolic alterations. Ann N Y Acad Sci. 2012;1264:87–102. doi:10.1111/j.1749-6632.2012.06597.x.
Armani A, Marzolla V, Fabbri A, Caprio M. Cellular mechanisms of MR regulation of adipose tissue physiology and pathophysiology. J Mol Endocrinol. 2015;55(2):R1–10. doi:10.1530/JME-15-0122. This review summarizes the functions of cellular mechanisms of MR in the adipocyte, discusses potential signaling pathways mediating MR action, and describes post-translational modifications regulating its activity.
Berger S, Bleich M, Schmid W, Greger R, Schutz G. Mineralocorticoid receptor knockout mice: lessons on Na + metabolism. Kidney Int. 2000;57(4):1295–8. doi:10.1046/j.1523-1755.2000.00965.x.
Bleich M, Warth R, Schmidt-Hieber M, Schulz-Baldes A, Hasselblatt P, Fisch D, et al. Rescue of the mineralocorticoid receptor knock-out mouse. Pflugers Arch - Eur J Physiol. 1999;438(3):245–54. doi:10.1007/s004240050906.
Lim HY, van den Brandt J, Fassnacht M, Allolio B, Herold MJ, Reichardt HM. Silencing of the mineralocorticoid receptor by ribonucleic acid interference in transgenic rats disrupts endocrine homeostasis. Mol Endocrinol. 2008;22(6):1304–11. doi:10.1210/me.2007-0417.
Ronzaud C, Loffing J, Bleich M, Gretz N, Grone HJ, Schutz G, et al. Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol. 2007;18(6):1679–87. doi:10.1681/ASN.2006090975.
Ronzaud C, Loffing J, Gretz N, Schutz G, Berger S. Inducible renal principal cell-specific mineralocorticoid receptor gene inactivation in mice. Am J Physiol Renal Physiol. 2011;300(3):F756–60. doi:10.1152/ajprenal.00728.2009.
Grossmann C, Benesic A, Krug AW, Freudinger R, Mildenberger S, Gassner B, et al. Human mineralocorticoid receptor expression renders cells responsive for nongenotropic aldosterone actions. Mol Endocrinol. 2005;19(7):1697–710. doi:10.1210/me.2004-0469.
Dooley R, Harvey BJ, Thomas W. Non-genomic actions of aldosterone: from receptors and signals to membrane targets. Mol Cell Endocrinol. 2012;350(2):223–34. doi:10.1016/j.mce.2011.07.019.
Munoz-Durango N, Barake MF, Letelier NA, Campino C, Fardella CE, Kalergis AM. Immune system alterations by aldosterone during hypertension: from clinical observations to genomic and non-genomic mechanisms leading to vascular damage. Curr Mol Med. 2013;13(6):1035–46.
Yogi A, Callera GE, O'Connor S, Antunes TT, Valinsky W, Miquel P, et al. Aldosterone signaling through transient receptor potential melastatin 7 cation channel (TRPM7) and its alpha-kinase domain. Cell Signal. 2013;25(11):2163–75. doi:10.1016/j.cellsig.2013.07.002.
Gros R, Ding Q, Sklar LA, Prossnitz EE, Arterburn JB, Chorazyczewski J, et al. GPR30 expression is required for the mineralocorticoid receptor–independent rapid vascular effects of aldosterone. Hypertension. 2011;57(3):442–51. doi:10.1161/hypertensionaha.110.161653.
Caroccia B, Seccia TM, Campos AG, Gioco F, Kuppusamy M, Ceolotto G, et al. GPER-1 and estrogen receptor-beta ligands modulate aldosterone synthesis. Endocrinology. 2014;155(11):4296–304. doi:10.1210/en.2014-1416.
Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology. 2013;154(11):4136–45. doi:10.1210/en.2013-1357.
Zhu P, Yuen JML, Sham KWY, Cheng CHK. GPER mediates the inhibitory actions of estrogen on adipogenesis in 3T3-L1 cells through perturbation of mitotic clonal expansion. Gen Comp Endocrinol. 2013;193:19–26. doi:10.1016/j.ygcen.2013.07.004.
Friso S, Carvajal CA, Fardella CE, Olivieri O. Epigenetics and arterial hypertension: the challenge of emerging evidence. Transl Res. 2015;165(1):154–65. doi:10.1016/j.trsl.2014.06.007.
Choi SW, Friso S. Epigenetics: a new bridge between nutrition and health. Adv Nutr. 2010;1(1):8–16. doi:10.3945/an.110.1004.
Demura M, Demura Y, Takeda Y, Saijoh K. Dynamic regulation of the angiotensinogen gene by DNA methylation, which is influenced by various stimuli experienced in daily life. Hypertens Res. 2015;38(8):519–27. doi:10.1038/hr.2015.42. This review highlights new evidences of epigenetic control of angiotensin gene (Agt) in adipose tissue. Agt gene, a RAAS gene, dynamically change its expression because exogenous and endogenous factors, as salt, glucocorticoids and mineralocorticoids in visceral adipose tissue.
Wang F, Demura M, Cheng Y, Zhu A, Karashima S, Yoneda T, et al. Dynamic CCAAT/enhancer binding protein-associated changes of DNA methylation in the angiotensinogen gene. Hypertension. 2014;63(2):281–8. doi:10.1161/HYPERTENSIONAHA.113.02303.
Howard B, Wang Y, Xekouki P, Faucz FR, Jain M, Zhang L, et al. Integrated analysis of genome-wide methylation and gene expression shows epigenetic regulation of CYP11B2 in aldosteronomas. J Clin Endocrinol Metab. 2014;99(3):E536–43. doi:10.1210/jc.2013-3495.
Robertson S, MacKenzie SM, Alvarez-Madrazo S, Diver LA, Lin J, Stewart PM, et al. MicroRNA-24 is a novel regulator of aldosterone and cortisol production in the human adrenal cortex. Hypertension. 2013;62(3):572–8. doi:10.1161/HYPERTENSIONAHA.113.01102.
Sober S, Laan M, Annilo T. MicroRNAs miR-124 and miR-135a are potential regulators of the mineralocorticoid receptor gene (NR3C2) expression. Biochem Biophys Res Commun. 2010;391(1):727–32. doi:10.1016/j.bbrc.2009.11.128.
Kemp JR, Unal H, Desnoyer R, Yue H, Bhatnagar A, Karnik SS. Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin-angiotensin system. J Mol Cell Cardiol. 2014;75:25–39. doi:10.1016/j.yjmcc.2014.06.008.
Misra M. Obesity pharmacotherapy: current perspectives and future directions. Curr Cardiol Rev. 2013;9(1):33–54.
Khorassani FE, Misher A, Garris S. Past and present of antiobesity agents: focus on monoamine modulators. Am J Health Syst Pharm. 2015;72(9):697–706. doi:10.2146/ajhp140034.
Costantino L, Barlocco D. New perspectives on the development of antiobesity drugs. Future Med Chem. 2015;7(3):315–36. doi:10.4155/fmc.14.167.
Gomez-Sanchez CE. What is the role of the adipocyte mineralocorticoid receptor in the metabolic syndrome? Hypertension. 2015;66(1):17–9. doi:10.1161/HYPERTENSIONAHA.115.05148.
Jing F, Mogi M, Horiuchi M. Role of renin–angiotensin–aldosterone system in adipose tissue dysfunction. Mol Cell Endocrinol. 2013;378(1–2):23–8. doi:10.1016/j.mce.2012.03.005.
Brown NJ. This is not Dr. Conn’s aldosterone anymore. Trans Am Clin Climatol Assoc. 2011;122:229–43.
Fontana V, de Faria AP, Oliveira-Paula GH, Silva PS, Biagi C, Tanus-Santos JE, et al. Effects of angiotensin-converting enzyme inhibition on leptin and adiponectin levels in essential hypertension. Basic Clin Pharmacol Toxicol. 2014;114(6):472–5. doi:10.1111/bcpt.12195.
Nedogoda SV, Ledyaeva AA, Chumachok EV, Tsoma VV, Mazina G, Salasyuk AS, et al. Randomized trial of perindopril, enalapril, losartan and telmisartan in overweight or obese patients with hypertension. Clin Drug Investig. 2013;33(8):553–61. doi:10.1007/s40261-013-0094-9.
Sarzani R, Guerra F, Mancinelli L, Buglioni A, Franchi E, Dessi-Fulgheri P. Plasma aldosterone is increased in class 2 and 3 obese essential hypertensive patients despite drug treatment. Am J Hypertens. 2012;25(7):818–26. doi:10.1038/ajh.2012.47.
Schrier RW. Aldosterone ‘escape’ vs ‘breakthrough’. Nat Rev Nephrol. 2010;6(2):61. doi:10.1038/nrneph.2009.228.
Marcus Y, Shefer G, Sasson K, Kohen F, Limor R, Pappo O, et al. Angiotensin 1–7 as means to prevent the metabolic syndrome: lessons from the fructose-fed rat model. Diabetes. 2013;62(4):1121–30. doi:10.2337/db12-0792.
Schuchard J, Winkler M, Stölting I, Schuster F, Vogt FM, Barkhausen J, et al. Lack of weight gain after angiotensin AT1 receptor blockade in diet-induced obesity is partly mediated by an angiotensin-(1–7)/Mas-dependent pathway. Br J Pharmacol. 2015;172(15):3764–78. doi:10.1111/bph.13172.
Azizi M, Amar L, Menard J. Aldosterone synthase inhibition in humans. Nephrol Dial Transplant. 2013;28(1):36–43. doi:10.1093/ndt/gfs388. This review summarizes recent in vitro and preclinical studies of CYP11B2 inhibitors, hormonal and haemodynamic effects, and highlights potential side-effects as a class.
Papillon JPN, Lou C, Singh AK, Adams CM, Ksander GM, Beil ME, et al. Discovery of N-[5-(6-chloro-3-cyano-1-methyl-1H-indol-2-yl)-pyridin-3-ylmethyl]-ethanesulfonamide, a cortisol-sparing CYP11B2 inhibitor that lowers aldosterone in human subjects. J Med Chem. 2015. doi:10.1021/acs.jmedchem.5b01545.
Strushkevich N, Gilep AA, Shen L, Arrowsmith CH, Edwards AM, Usanov SA, et al. Structural insights into aldosterone synthase substrate specificity and targeted inhibition. Mol Endocrinol. 2013;27(2):315–24. doi:10.1210/me.2012-1287. This paper reports the crystal structures of human aldosterone synthase in complex with a substrate deoxycorticosterone and an first generation CYP11B2 inhibitor fadrozole.
Cerny MA, Csengery A, Schmenk J, Frederick K. Development of CYP11B1 and CYP11B2 assays utilizing homogenates of adrenal glands: utility of monkey as a surrogate for human. J Steroid Biochem Mol Biol. 2015;154:197–205. doi:10.1016/j.jsbmb.2015.08.004.
Armani A, Cinti F, Marzolla V, Morgan J, Cranston GA, Antelmi A, et al. Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high-fat-diet-fed mice. FASEB J. 2014;28(8):3745–57. doi:10.1096/fj.13-245415.
Garg R, Kneen L, Williams GH, Adler GK. Effect of mineralocorticoid receptor antagonist on insulin resistance and endothelial function in obese subjects. Diabetes Obes Metab. 2014;16(3):268–72. doi:10.1111/dom.12224. This paper reports the results of the single clinical study of MR antagonists for the treatment of obesity, However only non-diabetic healthy obese were included in the study.
Peelman F, Zabeau L, Moharana K, Savvides SN, Tavernier J. 20 years of leptin: insights into signaling assemblies of the leptin receptor. J Endocrinol. 2014;223(1):T9–23. doi:10.1530/joe-14-0264.
Gertler A, Solomon G. Leptin-activity blockers: development and potential use in experimental biology and medicine. Can J Physiol Pharmacol. 2013;91(11):873–82. doi:10.1139/cjpp-2013-0012.
Okada-Iwabu M, Iwabu M, Ueki K, Yamauchi T, Kadowaki T. Perspective of small-molecule adipoR agonist for type 2 diabetes and short life in obesity. Diabetes Metab J. 2015;39(5):363–72. doi:10.4093/dmj.2015.39.5.363.
Lim S, Quon MJ, Koh KK. Modulation of adiponectin as a potential therapeutic strategy. Atherosclerosis. 2014;233(2):721–8. doi:10.1016/j.atherosclerosis.2014.01.051.
Tanabe H, Fujii Y, Okada-Iwabu M, Iwabu M, Nakamura Y, Hosaka T, et al. Crystal structures of the human adiponectin receptors. Nature. 2015;520(7547):312–6. doi:10.1038/nature14301. This paper reports the crystal structures of human AdipoR1 and AdipoR2, a novel class of receptor structures, with a seven-transmembrane helices arrangement, conformationally distinct from those of G-protein-coupled receptors.
Acknowledgments
This article was funded by CONICYT-FONDECYT grants No. 1130427, No. 1150437 and No. 1160695, IMII P09/016-F ICM, CORFO 13 CTi-21526-P1 grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Carvajal reports grants and personal fees from Fondecyt No. 1130427, from Fondecyt No. 1150437, from IMII P09/016-F ICM, and from CORFO 13CTI-21526-P1.
Dr. Baudrand reports grants and personal fees from Fondecyt No. 1130427, and from Fondecyt No. 1150437.
Dr. Fardella reports grants and personal fees from Fondecyt No. 1130427, from Fondecyt No. 1150437, from IMII P09/016-F ICM, and from CORFO 13CTI-21526-P1.
Dr. Lagos reports grants and personal fees from CORFO 13CTI-21526-P1.
Dr. Vecchiola reports grants and personal fees from Fondecyt No. 1130427, from Fondecyt No. 1150437, from IMII P09/016-F ICM, and from CORFO 13CTI-21526-P1.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hypertension and Obesity
Rights and permissions
About this article
Cite this article
Vecchiola, A., Lagos, C.F., Carvajal, C.A. et al. Aldosterone Production and Signaling Dysregulation in Obesity. Curr Hypertens Rep 18, 20 (2016). https://doi.org/10.1007/s11906-016-0626-9
Published:
DOI: https://doi.org/10.1007/s11906-016-0626-9