Abstract
Background
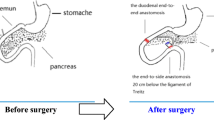
Duodenal-jejunal bypass (DJB) induces rapid and durable improvement in glucose and lipid metabolism. Besides bypassing the proximal gut, DJB also diverts bile acids (BAs) into the distal gut, increasing luminal and systemic BAs that are essential to metabolic homeostasis. The aim of this study is to evaluate whether bile diversion (BD) alone can recapitulate the effects of DJB on glucose and lipid metabolism.
Methods
BD, DJB and SHAM procedures were performed in a diabetic rat model induced by high-fat diet (HFD)/streptozotocin (STZ). Body weight, energy intake, blood glucose, serum hormones, insulin sensitivity, lipid profiles, and luminal and systemic BAs were measured postsurgery.
Results
BD reduced body weight, independently of energy intake, whereas DJB had no effects. Luminal and serum BAs were increased after both DJB and BD, and were higher after BD than DJB. During glucose tolerance test, both fasting and postprandial blood glucose concentrations were reduced with DJB and BD, and were lower after DJB than BD. Insulin sensitivity was improved after DJB, but remained unchanged after BD. Fasting and postprandial GLP-1 were equally increased after DJB and BD. Serum triglyceride and free fatty acids were decreased more after BD than DJB, while hepatic triglyceride storage was reduced more after DJB.
Conclusion
These observations indicate that BD, which increases luminal and systemic BAs and postprandial GLP-1, represents an important component of DJB in restoring glucose and lipid homeostasis in diabetic state. However, other mechanisms associated with DJB also appear to make complementary contributions to metabolic regulation.






Similar content being viewed by others
References
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244(5):741–9.
Ramos AC, Galvao Neto MP, de Souza YM, et al. Laparoscopic duodenal-jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI <30 kg/m2 (LBMI). Obes Surg. 2009;19(3):307–12.
Cohen RV, Schiavon CA, Pinheiro JS, et al. Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases. Surg Obes Relat Dis. 2007;3(2):195–7.
Cohen R, Caravatto PP, Correa JL, et al. Glycemic control after stomach-sparing duodenal-jejunal bypass surgery in diabetic patients with low body mass index. Surg Obes Relat Dis. 2012;8(4):375–80.
Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239(1):1–11.
Han H, Wang L, Du H, et al. Expedited biliopancreatic juice flow to the distal gut benefits the diabetes control after duodenal-jejunal bypass. Obes Surg. 2015;1.
Mazuy C, Helleboid A, Staels B, et al. Nuclear bile acid signaling through the farnesoid X receptor. Cell Mol Life Sci. 2015;72(9):1631–50.
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77.
Wu T, Bound MJ, Standfield SD, et al. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes Metab. 2013;15(5):474–7.
Manfredini G, Ermini M, Scopsi L, et al. Internal biliary diversion improves glucose tolerance in the rat. Am J Physiol. 1985;249(4 Pt 1):G519–27.
Kohli R, Setchell KD, Kirby M, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154(7):2341–51.
Wu T, Bound MJ, Standfield SD, et al. Effects of taurocholic acid on glycemic, glucagon-like peptide-1, and insulin responses to small intestinal glucose infusion in healthy humans. J Clin Endocrinol Metab. 2013;98(4):E718–22.
Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–20.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Bhutta HY, Rajpal N, White W, et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLoS One. 2015;10(3):e0122273.
Drozdzik M, Groer C, Penski J, et al. Protein abundance of clinically relevant multidrug transporters along the entire length of the human intestine. Mol Pharm. 2014;11(10):3547–55.
Kuipers F, Groen AK. FXR: the key to benefits in bariatric surgery? Nat Med. 2014;20(4):337–8.
Brown BD, Ammon HV. Effect of glucose on jejunal water and solute absorption in the presence of glycodeoxycholate and oleate in man. Dig Dis Sci. 1981;26(8):710–7.
Breen DM, Rasmussen BA, Kokorovic A, et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012;18(6):950–5.
Beglinger S, Drewe J, Schirra J, et al. Role of fat hydrolysis in regulating glucagon-like peptide-1 secretion. J Clin Endocrinol Metab. 2010;95(2):879–86.
Armstrong DN, Krenz HK, Modlin IM, et al. Bile salt inhibition of motility in the isolated perfused rabbit terminal ileum. Gut. 1993;34(4):483–8.
Cummings BP, Strader AD, Stanhope KL, et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138(7):2437–46.
Stefater MA, Sandoval DA, Chambers AP, et al. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology. 2011;141(3):939–49.
Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87(1):81–6.
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9.
Keyhani-Nejad F, Irmler M, Isken F, et al. Nutritional strategy to prevent fatty liver and insulin resistance independent of obesity by reducing glucose-dependent insulinotropic polypeptide responses in mice. Diabetologia. 2015;58(2):374–83.
Mueller M, Thorell A, Claudel T, et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol. 2015;62(6):1398–404.
Kaur A, Patankar JV, de Haan W, et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes. 2015;64(4):1168–79.
Everson GT. Steady-state kinetics of serum bile acids in healthy human subjects: single and dual isotope techniques using stable isotopes and mass spectrometry. J Lipid Res. 1987;28(3):238–52.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81270888/H0713, no. 81370496/H0308, no. 81300286/H0308), Natural Science Foundation of Shandong Province grants (no. ZR2009CM051), and the Taishan Scholar Foundation. Dr. T Wu is supported by a Royal Adelaide Hospital Research Committee Early Career Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing intersets.
Statement of Informed Consent
Does not apply.
Statement of Human and Animal Rights
All applicable institutional and national guidelines for the care and use of animals were followed.
Funding
This work was supported by the National Natural Science Foundation of China (no. 81270888/H0713, no. 81370496/H0308, no. 81300286/H0308), Natural Science Foundation of Shandong Province grants (no. ZR2009CM051), and the Taishan Scholar Foundation. Dr. T Wu is supported by a Royal Adelaide Hospital Research Committee Early Career Fellowship.
Rights and permissions
About this article
Cite this article
Zhang, X., Liu, T., Wang, Y. et al. Comparative Effects of Bile Diversion and Duodenal-Jejunal Bypass on Glucose and Lipid Metabolism in Male Diabetic Rats. OBES SURG 26, 1565–1575 (2016). https://doi.org/10.1007/s11695-015-1925-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1925-y