Summary
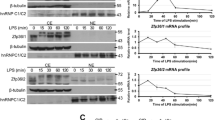
This study investigated the influence of silencing TRAF6 with shRNA on lipopolysaccharide (LPS)/toll-like receptor (TLR)-4 signaling pathway in vitro. Four plasmids (pGCsi-TRAF6-shRNA1, 2, 3, 4) containing different shRNA sequences were designed and synthesized. The proliferation of RAW264.7 cells after transfected with these plasmids was measured by MTT assay. Inflammatory cellular models were established by LPS stimulation. Levels of TNF-α, IL-1β and TGF-β1 in the supernatants, mRNA expressions of TRAF6, IL-6 and COX-2, protein expression of TRAF6 and translocation of NF-κB were assayed by ELISA, real-time quantitative PCR and Western blotting, respectively. The results showed that the TRAF6 gene knockdown by RNAi hardly inhibited the proliferation of RAW264.7 cells within 72 h. The mRNA and protein expression of TRAF6 was lower in the TRAF6-shRNA1, 2 groups than in the TRAF6-shRNA3, 4 groups. Therefore, pGCsi-TRAF6-shRNA1, 2 were selected for the subsequent experiments. Our results still showed that pGCsi-TRAF6-shRNA1, 2 could significantly reduce the production of pro-inflammatory cytokines and mediators including TNF-α, IL-1β, IL-6 and COX-2, and inhibit NF-κB nuclear translocation. Moreover, pGCsi-TRAF6-shRNA1, 2 could suppress the release of TGF-β1 at the protein level. It was concluded that the recombinant plasmid pTRAF6-shRNA can, to some extent, inhibit inflammatory response stimulated by LPS at the initial phase. TRAF6 may become the potential therapeutic target of many inflammation-related diseases.
Similar content being viewed by others
References
Doyle SL, O’Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol, 2006,72(9):1102–1113
Wu H, Arron JR. TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. Bioessays, 2003,25(11):1096–1105
Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone, 2007,40(2): 251–264
Kim KM, Kwon YG, Chung HT, et al. Methanol extract of cordyceps pruinosa inhibits in vitro and in vivo inflammatory mediators by suppressing NF-kappaB activation. Toxicol Appl Pharmacol, 2003,190(1):1–8
Feng C, Sheng SH, Rong YQ, et al. Construction and expression of mouse TRAF6-shRNA expression plasmid. World Chin J Digestol (Chinese), 2009,17(14):1406–1411
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods, 2001,25(4): 402–408
Hwang TH, Yoon BC, Jeong JS, et al. A single administration of adenoviral-mediated HGF cDNA permits survival of mice from acute hepatic failure. Life Sci, 2003,72(7):851–861
Qiu L, Song L, Yu Y, et al. Identification and expression of TRAF6 (TNF receptor-associated factor 6) gene in Zhikong Scallop Chlamys farreri. Fish Shellfish Immunol, 2009,26(3): 359–367
Belladonna ML, Vacca C, Volpi C, et al. IL-23 neutralization protects mice from gram-negative endotoxic shock. Cytokine, 2006,34(3–4):161–169
Kobayashi T, Walsh MC, Choi Y. The role of TRAF6 in signal transduction and the immune response. Microbes Infect, 2004,6(14):1333–1338
Loniewski KJ, Patial S, Parameswaran N. Sensitivity of TLR4-and-7-induced NF-κB1 p105-TPL2-ERK pathway to TNF-receptor-associated-factor-6 revealed by RNAi in mouse macrophages. Mol Immunol, 2007,44(15):3715–3723
Yang YJ, Chen W, Carrigan SO, et al. TRAF6 specifically contributes to Fcepsilon RI-mediated cytokine production but not mast cell degranulation. J Biol Chem, 2008,283(46): 32110–32118
Machado FS, Esper L, Dias A, et al. Native and aspirin-triggered lipoxins control innate immunity by inducing proteasomal degradation of TRAF6. J Exp Med, 2009, 206(11):2573
Lomaga MA, Henderson JT, Elia AJ, et al. TNF receptor-associated factor-6 (TRAF6) deficiency results in exencephaly and is required for apoptosis within the developing CNS. J Neurosci, 2000,20(19):7384–7393
Tian X, Zhang P, Zamek-Gliszczynski MJ, et al. Knocking down transport: applications of RNA interference in the study of drug transport proteins. Drug Metab Rev, 2005,37(4):705–723
George J, Tsutsumi M. siRNA-mediated knockdown of connective tissue growth factor prevents N-nitrosodimethylamine-induced hepatic fibrosis in rats. Gene Ther, 2007,14(10):790–803
Song E, Lee SK, Wang J, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med, 2003,9(3): 347–351
Kim KH, Kim HC, Hwang MY et al. The antifibrotic effect of TGF-beta1 siRNAs in murine model of liver cirrhosis. Biochem Biophys Res Commun, 2006,343(4): 1072–1078
Schwabe RF, Brenner DA. Mechanisms of liver injury I. TNF-alpha induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointes Liver Physiol, 2006, 290(4):G583–589
Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J Clin Invest, 2002,109(1):41–50
Otsuka M, Negishi Y, Aramaki Y. Involvement of phosphatidylinositol-3-kinase and ERK pathways in the production of TGF-β1 by macrophages treated with liposomes composed of phosphatidylserine. FEBS Lett, 2007, 581(2):325–330
Viatour P, Merville MP, Bours V, et al. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci, 2005,30(1):43–52
Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol, 2006,6(2):111–130
Aggarwal BB, Shishodia S, Sandur SK, et al. Inflammation and cancer: how hot is the link? Biochem Pharmacol, 2006,72(11):1605–1621
Tsatsanis C, Androulidaki A, Venihaki M, et al. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol, 2006,38(10):1654–166
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, F., He, S., Qiu, R. et al. Influence of silencing TRAF6 with shRNA on LPS/TLR4 signaling in vitro . J. Huazhong Univ. Sci. Technol. [Med. Sci.] 30, 278–284 (2010). https://doi.org/10.1007/s11596-010-0343-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-010-0343-6