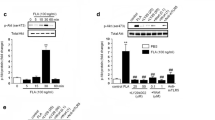
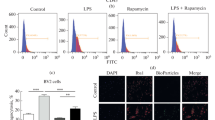
Abstract
Removal of apoptotic cells is an essential process for normal development and tissue maintenance. Importantly, apoptotic cells stimulate their phagocytosis by macrophages while actively suppressing inflammatory responses. Growth arrest specific gene 6 (Gas6) is involved in this process, bridging phosphatidylserine residues on the surface of apoptotic cells to the Axl/Mer family of tyrosine kinases which stimulate phagocytosis. Animals with mutations or loss of these receptors exhibit phenotypes reflective of impaired phagocytosis and a hyperactive immune response. We report that Gas6 induces phagocytosis in microglia through a novel non-classical phagocytic mechanism. Gas6 stimulates a type-II-related phagocytic response, but requires Vav phosphorylation and Rac activation, distinguishing it from the classical type II mechanism. Importantly, Gas6 suppressed lipopolysaccharide-induced expression of the inflammatory molecules IL-1β and iNOS. Gas6 inhibited iNOS expression through suppression of promoter activity. The present data provide direct evidence for the role of Gas6 receptors in mediating an anti-inflammatory response to ligands found on apoptotic cells with the simultaneous stimulation of phagocytosis. These data provide a mechanistic explanation for the phenotype observed in animals lacking Axl/Mer receptors.








Similar content being viewed by others
References
Aderem A, Underhill DM (1999) Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17:593–623
Bhat NR, Feinstein DL, Shen Q, Bhat AN (2002) p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J Biol Chem 277:29584–29592
Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM (1997) Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem 272:26159–26165
Bustelo XR (2001) Vav proteins, adaptors and cell signaling. Oncogene 20:6372–6381
Camenisch TD, Koller BH, Earp HS, Matsushima GK (1999) A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol 162:3498–3503
Caron E, Hall A (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717–1721
Combs CK, Karlo JC, Kao SC, Landreth GE (2001) beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci 21:1179–1188
Cvetanovic M, Ucker DS (2004) Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol 172:880–889
D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D (2000) Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9:645–651
Fadok VA, Chimini G (2001) The phagocytosis of apoptotic cells. Semin Immunol 13:365–372
Fadok VA, Henson PM (2003) Apoptosis: giving phosphatidylserine recognition an assist-with a twist. Curr Biol 13:R655–R657
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101:890–898
Funakoshi H, Yonemasu T, Nakano T, Matumoto K, Nakamura T (2002) Identification of Gas6, a putative ligand for Sky and Axl receptor tyrosine kinases, as a novel neurotrophic factor for hippocampal neurons. J Neurosci Res 68:150–160
Goruppi S, Ruaro E, Schneider C (1996) Gas6, the ligand of Axl tyrosine kinase receptor, has mitogenic and survival activities for serum starved NIH3T3 fibroblasts. Oncogene 12:471–480
Greenberg S (1995) Signal transduction of phagocytosis. Trends Cell Biol 5:93–99
Greenberg S (1999) Modular components of phagocytosis. J Leukoc Biol 66:712–717
Greenberg S, Chang P, Silverstein SC (1993) Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med 177:529–534
Hall MO, Obin MS, Heeb MJ, Burgess BL, Abrams TA (2005) Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp Eye Res 81:581–591
Hasanbasic I, Cuerquis J, Varnum B, Blostein MD (2004) Intracellular signaling pathways involved in Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol 287:H1207–H1213
Henson PM, Bratton DL, Fadok VA (2001) Apoptotic cell removal. Curr Biol 11:R795–R805
Hirt UA, Leist M (2003) Rapid, noninflammatory and PS-dependent phagocytic clearance of necrotic cells. Cell Death Differ 10:1156–1164
Isberg RR, Hamburger Z, Dersch P (2000) Signaling and invasin-promoted uptake via integrin receptors. Microbes Infect 2:793–801
Ishimoto Y, Ohashi K, Mizuno K, Nakano T (2000) Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem (Tokyo) 127:411–417
Koenigsknecht J, Landreth G (2004) Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci 24:9838–9846
Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW (1993) Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol 150:2659–2667
Lemke G, Lu Q (2003) Macrophage regulation by Tyro 3 family receptors. Curr Opin Immunol 15:31–36
Li S, Yamauchi A, Marchal CC, Molitoris JK, Quilliam LA, Dinauer MC (2002) Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J Immunol 169:5043–5051
Lu Q, Lemke G (2001) Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 293:306–311
Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, Earp HS, Goff SP, Lemke G (1999) Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 398:723–728
Mahajan NP, Earp HS (2003) An SH2 domain-dependent, phosphotyrosine-independent interaction between Vav1 and the Mer receptor tyrosine kinase: a mechanism for localizing guanine nucleotide-exchange factor action. J Biol Chem 278:42596–42603
Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K (1996) Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 271:30022–30027
Nakano T, Ishimoto Y, Kishino J, Umeda M, Inoue K, Nagata K, Ohashi K, Mizuno K, Arita H (1997) Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J Biol Chem 272:29411–29414
Newman SL, Mikus LK, Tucci MA (1991) Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J Immunol 146:967–974
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318
Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM (2002) Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr Biol 12:1413–1418
Pittoni V, Valesini G (2002) The clearance of apoptotic cells: implications for autoimmunity. Autoimmun Rev 1:154–161
Prieto AL, Weber JL, Tracy S, Heeb MJ, Lai C (1999) Gas6, a ligand for the receptor protein-tyrosine kinase Tyro-3, is widely expressed in the central nervous system. Brain Res 816:646–661
Prieto AL, Weber JL, Lai C (2000) Expression of the receptor protein-tyrosine kinases Tyro-3, Axl, and mer in the developing rat central nervous system. J Comp Neurol 425:295–314
Ravichandran KS (2003) “Recruitment signals” from apoptotic cells: invitation to a quiet meal. Cell 113:817–820
Savill J (1997) Recognition and phagocytosis of cells undergoing apoptosis. Br Med Bull 53:491–508
Schneider C, King RM, Philipson L (1988) Genes specifically expressed at growth arrest of mammalian cells. Cell 54:787–793
Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK (2001) Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411:207–211
Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF et al (1995) The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 80:661–670
Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ (2003) Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299:1743–1747
Streit WJ, Mrak RE, Griffin WS (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 1:14
Takahashi K, Rochford CD, Neumann H (2005) Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 201:647–657
Turner M, Billadeau DD (2002) VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat Rev Immunol 2:476–486
Turner M, Mee PJ, Walters AE, Quinn ME, Mellor AL, Zamoyska R, Tybulewicz VL (1997) A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity 7:451–460
Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, Hunt RW, Trail G, Clogston C, Toso RJ et al (1995) Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature 373:623–626
Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I (1997) Immunosuppressive effects of apoptotic cells. Nature 390:350–351
Weldon DT, Rogers SD, Ghilardi JR, Finke MP, Cleary JP, O’Hare E, Esler WP, Maggio JE, Mantyh PW (1998) Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J Neurosci 18:2161–2173
Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G (2006) Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem 281:20842–20850
Wu Y, Tibrewal N, Birge RB (2006) Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol 16:189–197
Yagami T, Ueda K, Asakura K, Sakaeda T, Nakazato H, Kuroda T, Hata S, Sakaguchi G, Itoh N, Nakano T, Kambayashi Y, Tsuzuki H (2002) Gas6 rescues cortical neurons from amyloid beta protein-induced apoptosis. Neuropharmacology 43:1289–1296
Yagami T, Ueda K, Asakura K, Okamura N, Sakaeda T, Sakaguchi G, Itoh N, Hashimoto Y, Nakano T, Fujimoto M (2003) Effect of Gas6 on secretory phospholipase A(2)-IIA-induced apoptosis in cortical neurons. Brain Res 985:142–149
Acknowledgments
We would like to thank Dr. Hugh Perry for his constructive discussions leading to this work. We thank Dr. N. Bhat (Medical University of South Carolina) for providing us with the iNOS reporter construct. This work was supported by grants from the NIH (AG-20202 and AG-16740) and the Blanchette Hooker Rockefeller Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grommes, C., Lee, C.Y.D., Wilkinson, B.L. et al. Regulation of Microglial Phagocytosis and Inflammatory Gene Expression by Gas6 Acting on the Axl/Mer Family of Tyrosine Kinases. J Neuroimmune Pharmacol 3, 130–140 (2008). https://doi.org/10.1007/s11481-007-9090-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-007-9090-2