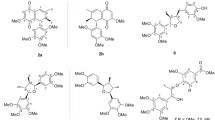
Abstract
Lignans are widely distributed in plants and exhibit significant pharmacological effects, including anti-tumor and antioxidative activities. Here, we describe the total synthesis of schizandriside (1), a compound we previously isolated from Saraca asoca by monitoring antioxidative activity using the 1,1-diphenyl-2-picrylhydrazyl radical scavenging assay. Starting from a tandem Michael-aldol reaction, the lignan skeleton was synthesized in 6 steps, including a cyclization step. To determine the stereochemistry of 1, we synthesized the natural product (±)-isolariciresinol (18) from alcohol 17. Comparison of the spectral data showed good agreement. Glycosylation was investigated using four different glycosyl donors. Only the Koenigs–Knorr condition using silver trifluoromethanesulfonate with 1,1,3,3-tetramethylurea provided the glycosylated product. Deprotection and purification using reverse-phase high-performance liquid chromatography gave schizandriside (1) and its diastereomer saracoside (2). Synthesized 1, 2 and 18 showed antioxidant activity with IC50 = 34.4, 28.8, 53.0 μM, respectively.






Similar content being viewed by others
References
Teponno RB, Kusari S, Spiteller M (2016) Recent advances in research on lignans and neolignans. Nat Prod Rep 33:1044–1092 (for a recent review)
Sadhu SK, Khatun A, Phattanawasin P, Ohtsuki T, Ishibashi M (2007) Lignan glycosides and flavonoids from Saraca asoca with antioxidant activity. J Nat Med 61:480–482
Sellars JD, Steel PG (2007) Advances in the synthesis of aryltetralin lignan lactones. Eur J Org Chem 23:3815–3828
Takani M, Ohya K, Takahashi K (1979) Studies on constituents of medicinal plants. XXII. Constituents of Schizandra nigra Max. (4). Chem Pharm Bull 27:1422–1425
Raffaelli B, Wähälä K, Hase T (2006) Asymmetric synthesis, stereochemistry and rearrangement reactions of naturally occurring 7′-hydroxylignano-9,9′-lactones. Org Biomol Chem 4:331–341
Zuo SJ, Li S, Yu RH, Zheng GX, Cao YX, Zhang SQ (2014) Discovery of novel 3-benzylquinazolin-4(3H)-ones as potent vasodilative agents. Bioorg Med Chem Lett 24:5597–5601
Brown CA (1970) Catalytic hydrogenation. V. The reaction of sodium borohydride with aqueous nickel salts. P-1 nickel bromide, a convenient, highly active nickel hydrogenation catalyst. J Org Chem 35:1900–1904
Brown CA, Ahuja VK (1973) Catalytic hydrogenation. VI. The reaction of sodium borohydride with nickel salts in ethanol solution. P-2 nickel, a highly convenient, new, selective hydrogenation catalyst with great sensitivity to substrate structure. J Org Chem 38:2226–2230
Jutiviboonsuk A, Zhang H, Tan GT, Ma C, Hung NV, Cuong NM, Bunyapraphatsara N, Soejarto DD, Fong HHS (2005) Bioactive constituents from roots of Bursera tonkinensis. Phytochemistry 66:2745–2751
Vogel K, Sterling J, Herzig Y, Nudelman A (1996) α-1-Tributyltin-O-2,3-bisacetyl-4,6-ethylidene-glucose as a convenient glycosidation reagent: an efficient synthesis of etoposide. Tetrahedron 52:3049–3056
Lundgren LN, Popoff T, Theander O (1981) Dilignol glycosides from needles of Picea abies. Phytochemistry 20:1967–1969
Sadhu SK, Okuyama E, Fujimoto H, Ishibashi M (2003) Separation of Leucas aspera, a medicinal plant of Bangladesh, guided by prostaglandin inhibitory and antioxidant activities. Chem Pharm Bull 51:595–598
Acknowledgements
This study was supported by KAKENHI Grant numbers 17H03992 and 15H04650, Suzuken Memorial Foundation, Takeda Science Foundation, Astellas Foundation for Research on Metabolic Disorders, the Naito Foundation, Strategic Priority Research Promotion Program, Chiba University, ‘Phytochemical Plant Molecular Sciences’, JSPS A3 Foresight Program, and a Workshop on Chirality at Chiba University (WCCU). This work was inspired by the international and interdisciplinary environment of the JSPS Core-to-Core Program ‘Asian Chemical Biology Initiative’.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sampei, M., Arai, M.A. & Ishibashi, M. Total syntheses of schizandriside, saracoside and (±)-isolariciresinol with antioxidant activities. J Nat Med 72, 651–654 (2018). https://doi.org/10.1007/s11418-018-1198-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-1198-6