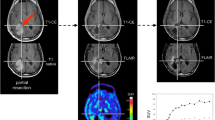
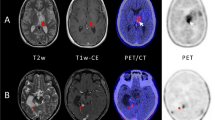
Abstract
Purpose
Cis-4-[18F]fluoro-D-proline (D-cis-[18F]FPro) has been shown to pass the intact blood-brain barrier and to accumulate in areas of secondary neurodegeneration and necrosis in the rat brain while uptake in experimental brain tumors is low. This pilot study explores the uptake behavior of D-cis-[18F]FPro in human brain tumors after multimodal treatment.
Procedures
In a prospective study, 27 patients with suspected recurrent brain tumor after treatment with surgery, radiotherapy, and/or chemotherapy (SRC) were investigated by dynamic positron emission tomography (PET) using D-cis-[18F]FPro (22 high-grade gliomas, one unspecified glioma, and 4 metastases). Furthermore, two patients with untreated lesions were included (one glioblastoma, one reactive astrogliosis). Data were compared with the results of PET using O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET) which detects viable tumor tissue. Tracer distribution, mean and maximum lesion-to-brain ratios (LBRmean, LBRmax), and time-to-peak (TTP) of the time activity curve (TAC) of tracer uptake were evaluated. Final diagnosis was determined by histology (n = 9), clinical follow-up (n = 10), or by [18F]FET PET (n = 10).
Results
D-cis-[18F]FPro showed high uptake in both recurrent brain tumors (n = 11) and lesions classified as treatment-related changes (TRC) only (n = 16) (LBRmean 2.2 ± 0.7 and 2.1 ± 0.6, n.s.; LBRmax 3.4 ± 1.2 and 3.2 ± 1.3, n.s.). The untreated glioblastoma and the lesion showing reactive astrogliosis exhibited low D-cis-[18F]FPro uptake. Distribution of [18F]FET and D-cis-[18F]FPro uptake was discordant in 21/29 cases indicating that the uptake mechanisms are different.
Conclusion
The high accumulation of D-cis-[18F]FPro in pretreated brain tumors and TRC supports the hypothesis that tracer uptake is related to cell death. Further studies before and after therapy are needed to assess the potential of D-cis-[18F]FPro for treatment monitoring.





Similar content being viewed by others
References
Langen KJ, Hamacher K, Bauer D, Bröer S, Pauleit D, Herzog H, Floeth F, Zilles K, Coenen HH (2005) Preferred stereoselective transport of the D-isomer of cis-4-[18F]fluoro-proline at the blood-brain barrier. J Cereb Blood Flow Metab 25:607–616
Langen KJ, Salber D, Hamacher K, Stoffels G, Reifenberger G, Pauleit D, Coenen HH, Zilles K (2007) Detection of secondary thalamic degeneration after cortical infarction using cis-4-18F-fluoro-D-proline. J Nucl Med 48:1482–1491
Geisler S, Willuweit A, Schroeter M, Zilles K, Hamacher K, Galldiks N, Shah NJ, Coenen HH, Langen KJ (2013) Detection of remote neuronal reactions in the thalamus and Hippocampus induced by rat glioma using the PET tracer cis-4-[18F]fluoro-D-proline. J Cereb Blood Flow Metab 33:724–731
Geisler S, Ermert J, Stoffels G, Willuweit A, Galldiks N, Filss C, Shah N, Coenen H, Langen KJ (2014) Isomers of 4-[18F]fluoro-proline: Radiosynthesis, biological evaluation and results in humans using PET. Curr Radiopharm 7:123–132
Wang X, Feng H, Zhao S, Xu J, Wu X, Cui J, Zhang Y, Qin Y, Liu Z, Gao T, Gao Y, Zeng W (2017) SPECT and PET radiopharmaceuticals for molecular imaging of apoptosis: from bench to clinic. Oncotarget 8:20476–20495
Allen AM, Ben-Ami M, Reshef A, Steinmetz A, Kundel Y, Inbar E, Djaldetti R, Davidson T, Fenig E, Ziv I (2012) Assessment of response of brain metastases to radiotherapy by PET imaging of apoptosis with 18F-ML-10. Eur J Nucl Med Mol Imaging 39:1400–1408
Oborski MJ, Laymon CM, Lieberman FS, Drappatz J, Hamilton RL, Mountz JM (2014) First use of 18F-labeled ML-10 PET to assess apoptosis change in a newly diagnosed glioblastoma multiforme patient before and early after therapy. Brain Behav 4:312–315
Doss M, Kolb HC, Walsh JC, Mocharla V, Fan H, Chaudhary A, Zhu Z, Alpaugh RK, Lango MN, Yu JQ (2013) Biodistribution and radiation dosimetry of 18F-CP-18, a potential apoptosis imaging agent, as determined from PET/CT scans in healthy volunteers. J Nucl Med 54:2087–2092
Oborski MJ, Laymon CM, Qian Y, Lieberman FS, Nelson AD, Mountz JM (2014) Challenges and approaches to quantitative therapy response assessment in glioblastoma Multiforme using the novel apoptosis positron emission tomography tracer F-18 ML-10. Transl Oncol 7:111–119
Langen KJ, Stoffels G, Filss C, Heinzel A, Stegmayr C, Lohmann P, Willuweit A, Neumaier B, Mottaghy FM, Galldiks N (2017) Imaging of amino acid transport in brain tumours: positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine (FET). Methods 130:124–134
Stegmayr C, Bandelow U, Oliveira D, Lohmann P, Willuweit A, Filss C, Galldiks N, Lübke JHR, Shah NJ, Ermert J, Langen KJ (2017) Influence of blood-brain barrier permeability on O-(2-18F-fluoroethyl)-L-tyrosine uptake in rat gliomas. Eur J Nucl Med Mol Imaging 44:408–416
Stegmayr C, Oliveira D, Niemietz N, Willuweit A, Lohmann P, Galldiks N, Shah NJ, Ermert J, Langen KJ (2017) Influence of bevacizumab on blood-brain barrier permeability and O-(2-18F-Fluoroethyl)-l-tyrosine uptake in rat gliomas. J Nucl Med 58:700–705
Langen KJ, Galldiks N, Hattingen E, Shah NJ (2017) Advances in neuro-oncology imaging. Nat Rev Neurol 13:279–289
Galldiks N, Stoffels G, Filss C, Rapp M, Blau T, Tscherpel C, Ceccon G, Dunkl V, Weinzierl M, Stoffel M, Sabel M, Fink GR, Shah NJ, Langen KJ (2015) The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro-Oncology 17:1293–1300
Pöpperl G, Gotz C, Rachinger W et al (2004) Value of O-(2-[18F]fluoroethyl)- L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 31:1464–1470
Galldiks N, Dunkl V, Stoffels G, Hutterer M, Rapp M, Sabel M, Reifenberger G, Kebir S, Dorn F, Blau T, Herrlinger U, Hau P, Ruge MI, Kocher M, Goldbrunner R, Fink GR, Drzezga A, Schmidt M, Langen KJ (2015) Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging 42:685–695
Galldiks N, Stoffels G, Filss CP, Piroth MD, Sabel M, Ruge MI, Herzog H, Shah NJ, Fink GR, Coenen HH, Langen KJ (2012) Role of O-(2-18F-Fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med 53:1367–1374
Galldiks N, Stoffels G, Ruge MI, Rapp M, Sabel M, Reifenberger G, Erdem Z, Shah NJ, Fink GR, Coenen HH, Langen KJ (2013) Role of O-(2-18F-fluoroethyl)-L-tyrosine PET as a diagnostic tool for detection of malignant progression in patients with low-grade glioma. J Nucl Med 54:2046–2054
Hamacher K (1999) Synthesis of N.C.A. Cis- and trans-4-[18F]fluoro-l-proline, radiotracers for PET-investigation of disordered matrix protein synthesis. J Label Compd Radiopharm 42:1135–1144
Wester HJ, Herz M, Senekowitsch-Schmidtke R et al (1992) Preclinical evaluation of 4-[18F]fluoroprolines: diastereomeric effect on metabolism and uptake in mice. Nucl Med Biol 26:259–265
Langen KJ, Bartenstein P, Boecker H, Brust P, Coenen HH, Drzezga A, Grünwald F, Krause BJ, Kuwert T, Sabri O, Tatsch K, Weber WA, Schreckenberger M (2011) German guidelines for brain tumour imaging by PET and SPECT using labelled amino acids. Nuklearmedizin 50:167–173
Langen KJ, Hamacher K, Weckesser M, Floeth F, Stoffels G, Bauer D, Coenen HH, Pauleit D (2006) O-(2-[18F]fluoroethyl)-L-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol 33:287–294
Unterrainer M, Vettermann F, Brendel M, Holzgreve A, Lifschitz M, Zähringer M, Suchorska B, Wenter V, Illigens BM, Bartenstein P, Albert NL (2017) Towards standardization of 18F-FET PET imaging: do we need a consistent method of background activity assessment? EJNMMI Res 7:48
Rapp M, Heinzel A, Galldiks N, Stoffels G, Felsberg J, Ewelt C, Sabel M, Steiger HJ, Reifenberger G, Beez T, Coenen HH, Floeth FW, Langen KJ (2013) Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med 54:229–235
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Broer A, Tietze N, Kowalczuk S et al (2006) The orphan transporter v7-3 (slc6a15) is a Na+−dependent neutral amino acid transporter (B0AT2). Biochem J 393:421–430
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by both the local ethics committee and federal authorities, and all patients gave written informed consent before each PET investigation and the use of the data for scientific evaluations.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed written consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Verger, A., Stoffels, G., Galldiks, N. et al. Investigation of cis-4-[18F]Fluoro-D-Proline Uptake in Human Brain Tumors After Multimodal Treatment. Mol Imaging Biol 20, 1035–1043 (2018). https://doi.org/10.1007/s11307-018-1197-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-018-1197-8