Abstract
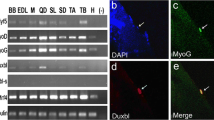
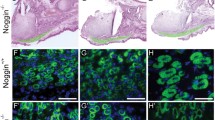
The t(2;13) chromosomal translocation is found in the majority of human alveolar rhabdomyosarcomas (RMS). The resulting PAX3-FKHR fusion protein contains PAX3 DNA-binding domains fused to the potent transactivation domain of FKHR, suggesting that PAX3-FKHR functions to deregulate PAX3-specific target genes and signaling pathways. We previously developed transgenic mice expressing PAX3-FKHR under the control of mouse Pax3 regulatory sequences to test this hypothesis. We reported that PAX3-FKHR interferes with normal Pax3 developmental functions, with mice exhibiting neural tube and neural crest abnormalities that mimic those found in Pax3-deficient Splotch mice. Here we expanded those studies to show that developmental expression of PAX3-FKHR results in aberrant myogenesis in the developing somites and neural tube, leading to ectopic skeletal muscle formation in the mature spinal cord. Gene expression profiling indicated that PAX3-FKHR expression in the developing neural tube induces a myogenic pattern of gene expression at the expense of the normal neurogenic program. Somite defects in PAX3-FKHR transgenic animals resulted in skeletal malformations that included rib fusions and mis-attachments. As opposed to the neural tube defects, the severity of the rib phenotype was rescued by reducing Pax3 levels through mating with Splotch mice. Embryos from the transgenic line expressing the highest levels of PAX3-FKHR had severe neural tube defects, including exencephaly, and almost half of the embryos died between gestational ages E13.5-E15.5. Nearly all of the embryos that survived to term died after birth due to severe spina bifida, rather than the absence of a muscular diaphragm. These studies reveal a prominent role for PAX3-FKHR in disrupting Pax3 functions and in deregulating skeletal muscle development, suggesting that this fusion protein plays a critical role in the pathogenesis of␣alveolar RMS by influencing the commitment␣and differentiation of the myogenic cell lineage.
Similar content being viewed by others
References
Anastasi S, Giordano S, Sthandier O, Gambarotta G, Maione R, Comoglio P, Amati P (1997) A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J Cell Biol 137:1057–1068
Anderson MJ, Shelton GD, Cavenee WK, Arden KC (2001) Embryonic expression of the tumor-associated PAX3-FKHR fusion protein interferes with the developmental functions of Pax3. Proc Natl Acad Sci USA 98:1589–1594
Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC (1998) Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics 47:187–199
Arden KC, Anderson MJ, Finckenstein FG, Czekay S, Cavenee WK (1996) Detection of the t(2;13) chromosomal translocation in alveolar rhabdomyosarcoma using the reverse transcriptase-polymerase chain reaction. Genes Chromosomes Cancer 16:254–260
Auerbach R (1954) Analysis of the developmental effects of a lethal mutation in the house mouse. J Exp Zool 127:305–329
Bennicelli JL, Fredericks WJ, Wilson RB, Rauscher FJ III, Barr FG (1995) Wild type PAX3 protein and the PAX3-FKHR fusion protein of alveolar rhabdomyosarcoma contain potent, structurally distinct transcriptional activation domains. Oncogene 11:119–130
Braun T, Rudnicki MA, Arnold H-H, Jaenisch R (1992) Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71:369–382
Brown CB, Engleka KA, Wenning J, Min Lu M, Epstein JA (2005) Identification of a hypaxial somite enhancer element regulating Pax3 expression in migrating myoblasts and characterization of hypaxial muscle Cre transgenic mice. Genesis 41:202–209
Conlon R (1994) In situ hybridization of whole mount embryos with RNA probes. In: Hogan B, Beddington R, Costantini F, Lacy E (eds) Manipulating the mouse embryo: a laboratory manual, 2nd edn. Cold␣Spring Harbor Laboratory Press, New York, pp␣360–362
Conway SJ, Henderson DJ, Kirby ML, Anderson RH, Copp AJ (1997) Development of a lethal congenital heart defect in the splotch (Pax3) mutant mouse. Cardiovascular Res 36:163–173
Daston G, Lamar E, Olivier M, Goulding M (1996) Pax-3 is necessary for migration but not differentiation of limb muscle precursors in the mouse. Development 122:1017–1027
Davis RJ, D’Cruz CM, Lovell MA, Biegel JA, Barr FG (1994) Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer Res 54:2869–2872
Delfini M-C, Duprez D (2004) Ectopic Myf5 or MyoD prevents the neuronal differentiation program in addition to inducing skeletal muscle differentiation, in the chick neural tube. Development 131:713–723
Epstein JA, Lam P, Jepeal L, Maas RL, Shapiro DN (1995) Pax3 inhibits myogenic differentiation of cultured myoblast cells. J Biol Chem 270:11719–11722
Epstein JA, Shapiro DN, Cheng J, Lam PYP, Maas RL (1996) Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc Natl Acad Sci USA 93:4213–4218
Ferracini R, Olivero M, Di Renzo MF, Martano M, De Giovanni C, Nanni P, Basso G, Scotlandi K, Lollini P-L, Comoglio PM (1996) Retrogenic expression of the MET proto-oncogene correlates with the invasive phenotype of human rhabdomyosarcomas. Oncogene 12:1697–1705
Franz T (1989) Persistent truncus arteriosus in the splotch mutant mouse. Anat Embryol 180:457–464
Fredericks WJ, Galili N, Mukhopadhyay S, Rovera G, Bennicelli J, Barr FG, Rauscher FJ III (1995) The PAX3-FKHR fusion protein created by the t(2;13) translocation in alveolar rhabdomyosarcomas is a more potent transcriptional activator than PAX3. Mol Cell Biol 15:1522–1535
Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ III, Emanuel BS, Rovera G, Barr FG (1993) Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet 5:230–235
Garrick D, Fiering S, Martin DIK, Whitelaw E (1998) Repeat-induced gene silencing in mammals. Nat Genet 18:56–59
Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P (1991) Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J 10:1135–1147
Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH (1993) Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364:501–506
Hosack DA, Dennis G Jr, Sherman BT, Lane HC, Lempicki RA (2003) Identifying biological themes within lists of genes with EASE. Genome Biol 4: R70
James AC, Veitch JG, Zareh AR, Triche T (2004) Sensitivity and specificity of five abundance estimators for high-density oligonucleotide microarrays. Bioinformatics 20:1060–1065
Kablar B, Rudnicki MA (1999) Development in the absence of skeletal muscle results in the sequential ablation of motor neurons from the spinal cord to the brain. Dev Biol 208:93–109
Keller C, Arenkiel BR, Coffin CM, El-Bardeesy N, DePinho RA, Capecchi MR (2004a) Alveolar rhabdomyosarcomas in conditional Pax3:Fkhr mice: cooperativity of Ink4a/ARF and Trp53 loss of function. Genes Dev 18:2614–2626
Keller C, Hansen MS, Coffin CM, Capecchi MR (2004b) Pax3:Fkhr interferes with embryonic Pax3 and Pax7 function: implications for alveolar rhabdomyosarcoma cell of origin. Genes Dev 18:2608–2613
Khan J, Bittner ML, Saal LH, Teichmann U, Azorsa DO, Gooden GC, Pavan WJ, Trent JM, Meltzer PS (1999) cDNA microarrays detect activation of a myogenic transcription program by the PAX3-FKHR fusion oncogene. Proc Natl Acad Sci USA 96:13264–13269
Lagutina I, Conway SJ, Sublett J, Grosveld GC (2002) Pax3-FKHR knock-in mice show developmental aberrations but do not develop tumors. Mol Cell Biol 22:7204–7216
Lam PYP, Sublett JE, Hollenbach AD, Roussel MF (1999) The oncogenic potential of the Pax3-FKHR fusion protein requires the Pax3 homeodomain recognition helix but not the Pax3 paired-box DNA binding domain. Mol Cell Biol 19:594–601
Liu LN, Dias P, Houghton PJ (1998) Mutation of Thr115 in MyoD positively regulates function in murine fibroblasts and human rhabdomyosarcoma cells. Cell Growth Diff 9:699–711
Milewski RC, Chi NC, Li J, Brown C, Lu MM, Epstein JA (2004) Identification of minimal enhancer elements sufficient for Pax3 expression in neural crest and implication of Tead2 as a regulator of Pax3. Development 131:829–837
Natoli TA, Ellsworth MK, Wu C, Gross KW, Pruitt SC (1997) Positive and negative DNA sequence elements are required to establish the pattern of Pax3 expression. Development 124:617–626
Peter M, Gilbert E, Delattre O (2001) A multiplex real-time PCR assay for the detection of gene fusions observed in solid tumors. Lab Invest 81:905–912
Relaix F, Polimeni M, Rocancourt D, Ponzetto C, Schafer BW, Buckingham M (2003) The transcriptional activator PAX3-FKHR rescues the defects of Pax3 mutant mice but induces a myogenic gain-of-function phenotype with ligand-independent activation of Met signaling in vivo. Genes Dev 17:2950–2965
Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT, Jacks T (1995) A subset of p53-deficient embryos exhibit exencephaly. Nat Genet 10:175–180
Sassoon DA, Lyons G, Wright WE, Lin V, Lassar A, Weintraub H, Buckingham M (1989) Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature 341:303–307
Sorensen PHB, Lynch JC, Qualman SJ, Tirabosco R, Lim JF, Maurer HM, Bridge JA, Crist WM, Triche TJ, Barr FG (2002) PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the Children’s Oncololgy Group. J Clin Oncol 20:2672–2679
Sublett JE, Jeon I-S, Shapiro DN (1995) The alveolar rhabdomyosarcoma PAX3/FKHR fusion protein is a transcriptional activator. Oncogene 11:545–552
Takayama H, LaRochelle WJ, Anver M, Bockman DE, Merlino G (1996) Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci USA 93:5866–5871
Tapscott SJ, Thayer MJ, Weintraub H (1993) Deficiency in rhabdomyosarcomas of a factor required for MyoD activity and myogenesis. Science 259:1450–1453
Tremblay P, Dietrich S, Mericskay M, Schubert FR, Li Z, Paulin D (1998) A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev Biol 203:49–61
Triche TJ, Sorensen PHB (2002) Molecular pathology of pediatric malignancies. In: Pizzo PA, Poplack DG (eds) Principles and practice of pediatric oncology, 4th edn. Lippincott Williams & Wilkins, Philadelphia, pp 161–204
Wilkinson DG (1992) Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson DG (eds) In situ hybridization: a practical approach. Oxford University Press, New York, pp 75–83
Yamamoto Y, Livet J, Pollock RA, Garces A, Arce V, deLapeyriere O, Henderson CE (1997) Hepatocyte growth factor (HGF/SF) is a muscle-derived survival factor for a subpopulation of embryonic motoneurons. Development 124:2903–2913
Yang X-M, Vogan K, Gros P, Park M (1996) Expression of the met receptor tyrosine kinase in muscle progenitor cells in somites and limbs is absent in Splotch mice. Development 122:2163–2171
Acknowledgements
We thank Hiroyuki Shimada and Sue Ann Phung for tissue sectioning; Patricia Reid for electron microscopy; Betty Schaub and Sitara Waidyaratne for microarray processing; Andrew Lassar, Morag Park and Peter Gruss for the mouse MyoD, c-met and Pax3 cDNA constructs, respectively; Violette Shahbazian and Antonia Boyer for excellent technical assistance; Diane Shelton, George McNamara and Nicole Tedeschi for helpful discussions. This work was supported by a Faculty Research Career Development Award (MJA) from the Saban Research Institute at Childrens Hospital Los Angeles. WKC is a Fellow of the National Foundation for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Finckenstein, F.G., Davicioni, E., Osborn, K.G. et al. Transgenic mice expressing PAX3-FKHR have multiple defects in muscle development, including ectopic skeletal myogenesis in the developing neural tube. Transgenic Res 15, 595–614 (2006). https://doi.org/10.1007/s11248-006-9011-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-006-9011-9