Abstract
Purpose
Over 25 million Americans reported having daily pain and between 5 and 8 million Americans used opioids to treat chronic pain in 2012. This is the first systematic review with meta-analysis to determine the effects of long-term opioid use on the Physical Component Summary (PCS) score and Mental Component Summary (MCS) scores of a Health-Related Quality of Life instrument in adults without opioid use disorder.
Methods
The a priori eligibility criteria for the PubMed (MEDLINE), Scopus, and PsyINFO searches were (1) randomized controlled trial, (2) at least one opioid intervention group, (3) minimum of 4-week duration of opioid use, (4) comparative control group, and (5) adults ≥18 years that do not have dominant disease. The unit of analysis was the standardized mean difference effect size (Hedges’s g). All results were pooled using random-effects models.
Results
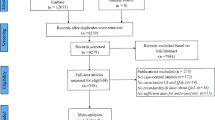
Of the 340 non-duplicate citations screened, 19 articles comprising 26 treatment comparisons and 6168 individuals (treatment n = 3160; comparators n = 3008 with duplicates removed) met the inclusion criteria for the systematic review. Thirteen treatment comparisons were available for the meta-analysis. Across all PCS analyses, small, statistically significant improvements were observed (opioid versus opioid only: g = 0.27, 95% CI 0.05–0.50, opioid versus placebo only: g = 0.18, 95% CI 0.08–0.28, and all studies combined: g = 0.22, 95% CI 0.11–0.32). There were small but not statistically significant changes on the MCS scores. Overall, high heterogeneity was present.
Conclusions
PCS scores improve with no change in MCS scores. However, long-term opioid trials are rare and only two trials included lasted longer than 1 year.







Similar content being viewed by others
References
Ballantyne, J. C., & Shin, N. S. (2008). Efficacy of opioids for chronic pain: A review of the evidence. The Clinical Journal of Pain, 24(6), 469–478.
Eriksen, J., Sjogren, P., Bruera, E., Ekholm, O., & Rasmussen, N. K. (2006). Critical issues on opioids in chronic non-cancer pain: An epidemiological study. Pain, 125(1–2), 172–179.
Institute of Medicine. (2011). Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press
Gaskin, D. J., & Richard, P. (2012). The economic costs of pain in the United States. The Journal of Pain: Official Journal of the American Pain Society, 13(8), 715–724.
Nahin, R. L. (2015). Estimates of pain prevalence and severity in adults: United States, 2012. The Journal of Pain: Official Journal of the American Pain Society, 16(8), 769–780.
Rosenblum, A., Marsch, L. A., Joseph, H., & Portenoy, R. K. (2008). Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Experimental and Clinical Psychopharmacology, 16(5), 405–416.
National Institute of Drug Abuse. What are Opioids? Prescription Drugs of Abuse 2014. https://www.drugabuse.gov/publications/research-reports/prescription-drugs/opioids/what-are-opioids, 2016.
Dowell, D., Haegerich, T. M., & Chou, R. (2016). CDC guideline for prescribing opioids for chronic pain—United States. JAMA, 315, 1624–1645.
Agency for Healthcare Research and Quality. The effectiveness and risks of long-term opioid treatment of chronic pain 2014. http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=1988&pageaction=displayproduct, 2016.
Centers for Disease Control and Prevention. Opioid painkiller prescribing 2014. CDC Vital Signs. http://www.cdc.gov/vitalsigns/opioid-prescribing, 2016.
Centers for Disease Control and Prevention. (2011). Vital signs: Overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR: Morbidity and Mortality Weekly Report, 60(43), 1487–1492.
Zgierska, A., Miller, M., & Rabago, D. (2012). Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA: The Journal of the American Medical Association, 307(13), 1377–1378.
Ballantyne, J. C., & Mao, J. (2003). Opioid therapy for chronic pain. The New England Journal of Medicine, 349(20), 1943–1953.
Centers for Disease Control and Prevention. HRQOL Concepts 2011. http://www.cdc.gov/hrqol/concept.htm, 2016.
Coons, S. J., Rao, S., Keininger, D. L., & Hays, R. D. (2000). A comparative review of generic quality-of-life instruments. Pharmacoeconomics, 17(1), 13–35.
Cook, D. J., Mulrow, C. D., & Haynes, R. B. (1997). Systematic reviews: Synthesis of best evidence for clinical decisions. Annals of Internal Medicine, 126(5), 376–380.
Garg, A. X., Hackman, D., & Tonelli, M. (2008). Systematic review and meta-analysis: When one study is just not enough. Clinical Journal of the American Society of Nephrology, 3(1), 253–260.
Skelly, A. C. (2014). Credibility matters: Mind the gap. Evid Based Spine Care Journal, 5(1), 2–5.
De Maeyer, J., Vanderplasschen, W., & Broekaert, E. (2010). Quality of life among opiate-dependent individuals: A review of the literature. International Journal on Drug Policy, 21(5), 364–380.
Ekstrom, M., Nilsson, F., Abernethy, A. A., & Currow, D. C. (2015). Effects of opioids on breathlessness and exercise capacity in chronic obstructive pulmonary disease. A systematic review. Annals of the American Thoracic Society, 12(7), 1079–1092.
Thakur, D., Dickerson, S., Kumar Bhutani, M., & Junor, R. (2015). Impact of prolonged-release oxycodone/naloxone on outcomes affecting patients’ daily functioning in comparison with extended-release tapentadol: A systematic review. Clinical Therapeutics, 37(1), 212–224.
Liberati, A., Altman, D. G., Tetzlaff, J., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ, 339, b2700.
Afilalo, M., & Morlion, B. (2013). Efficacy of tapentadol ER for managing moderate to severe chronic pain. Pain Physician, 16(1), 27–40.
Baron, R., Jansen, J. P., Binder, A., et al. (2015). Tolerability, safety, and quality of life with tapentadol prolonged release (pr) compared with oxycodone/naloxone PR in patients with severe chronic low back pain with a neuropathic component: A randomized, controlled, open-label, phase 3b/4 trial. Pain Practice. doi:10.1111/papr.12361.
Bennett, R. M., Schein, J., Kosinski, M. R., Hewitt, D. J., Jordan, D. M., & Rosenthal, N. R. (2005). Impact of fibromyalgia pain on health-related quality of life before and after treatment with tramadol/acetaminophen. Arthritis and Rheumatism, 53(4), 519–527.
Binsfeld, H., Szczepanski, L., Waechter, S., Richarz, U., & Sabatowski, R. (2010). a randomized study to demonstrate noninferiority of once-daily OROS® hydromorphone with twice-daily sustained-release oxycodone for moderate to severe chronic noncancer pain. Pain Practice: The Official Journal of World Institute of Pain, 10(5), 404–415.
Gajria, K., Kosinski, M., Schein, J., Kavanagh, S., & Dubois, D. (2008). health-related quality-of-life outcomes in patients treated with push-pull OROS hydromorphone versus Extended-release oxycodone for chronic hip or knee osteoarthritis pain: A randomized, open-label, parallel-group, multicenter study. The Patient, 1(3), 223–238.
Gordon, A., Callaghan, D., Spink, D., et al. (2010). Buprenorphine transdermal system in adults with chronic low back pain: A randomized, double-blind, placebo-controlled crossover study, followed by an open-label extension phase. Clinical Therapeutics, 32(5), 844–860.
Kalso, E., Simpson, K. H., Slappendel, R., Dejonckheere, J., & Richarz, U. (2007). Predicting long-term response to strong opioids in patients with low back pain: Findings from a randomized, controlled trial of transdermal fentanyl and morphine. BMC Medicine, 5, 39.
Allan, L., Richarz, U., Simpson, K., & Slappendel, R. (2005). Transdermal fentanyl versus sustained release oral morphine in strong-opioid naive patients with chronic low back pain. Spine, 30(22), 2484–2490.
Karlsson, M., & Berggren, A. C. (2009). Efficacy and safety of low-dose transdermal buprenorphine patches (5, 10, and 20 μg/h) versus prolonged-release tramadol tablets (75, 100, 150, and 200 mg) in patients with chronic osteoarthritis pain: A 12-week, randomized, open-label, controlled, parallel-group noninferiority study. Clinical Therapeutics, 31(3), 503–513.
Khoromi, S., Cui, L., Nackers, L., & Max, M. B. (2007). Morphine, nortriptyline and their combination vs. placebo in patients with chronic lumbar root pain. Pain, 130(1–2), 66–75.
Ma, K., Jiang, W., Zhou, Q., & Du, D. P. (2008). The efficacy of oxycodone for management of acute pain episodes in chronic neck pain patients. International Journal of Clinical Practice, 62(2), 241–247.
Miller, K., Yarlas, A., Wen, W., et al. (2013). Buprenorphine transdermal system and quality of life in opioid-experienced patients with chronic low back pain. Expert Opinion on Pharmacotherapy, 14(3), 269–277.
Nicholson, B., Ross, E., Sasaki, J., & Weil, A. (2006). Randomized trial comparing polymer-coated extended-release morphine sulfate to controlled-release oxycodone HCl in moderate to severe nonmalignant pain. Current Medical Research and Opinion, 22(8), 1503–1514.
Pedersen, L., Borchgrevink, P. C., Breivik, H. P., & Fredheim, O. M. (2014). A randomized, double-blind, double-dummy comparison of short- and long-acting dihydrocodeine in chronic non-malignant pain. Pain, 155(5), 881–888.
Peloso, P. M., Fortin, L., Beaulieu, A., Kamin, M., & Rosenthal, N. R. (2004). Analgesic efficacy and safety of tramadol/acetaminophen combination tablets (Ultracet®) in treatment of chronic low back pain: A multicenter, outpatient, randomized, double blind, placebo controlled trial. The Journal of Rheumatology, 31(12), 2454–2463.
Rauck, R. L., Bookbinder, S. A., Bunker, T. R., et al. (2007). A randomized, open-label, multicenter trial comparing once-a-day AVINZA® (morphine sulfate extended-release capsules) versus twice-a-day OxyContin® (oxycodone hydrochloride controlled-release tablets) for the treatment of chronic, moderate to severe low back pain: Improved physical functioning in the ACTION trial. Journal of Opioid Management, 3(1), 35–43.
Ruoff, G. E., Rosenthal, N., Jordan, D., Karim, R., & Kamin, M. (2003). Tramadol/acetaminophen combination tablets for the treatment of chronic lower back pain: A multicenter, randomized, double-blind, placebo-controlled outpatient study. Clinical Therapeutics, 25(4), 1123–1141.
Ueberall, M. A., Eberhardt, A., & Mueller-Schwefe G. H. H. (2016). Quality of life under oxycodone/naloxone, oxycodone, or morphine treatment for chronic low back pain in routine clinical practice. International Journal of General Medicine, 9, 39–51.
Watson, C. P., Moulin, D., Watt-Watson, J., Gordon, A., & Eisenhoffer, J. (2003). Controlled-release oxycodone relieves neuropathic pain: A randomized controlled trial in painful diabetic neuropathy. Pain, 105(1–2), 71–78.
Yarlas, A., Miller, K., Wen, W., et al. (2013). A randomized, placebo-controlled study of the impact of the 7-day buprenorphine transdermal system on health-related quality of life in opioid-naïve patients with moderate-to-severe chronic low back pain. Journal of Pain, 14(1), 14–23.
Hedges, L. V., & Olkin, I. (1985). Statistical methods for meta-analysis. San Deigo, CA: Academic Press.
Ahn SB, J. B. (2011). Incorporating quality scores in meta-analysis. Journal of Educational and Behavioral Statistics, 36(5), 555–585.
Wilson, D. Practical meta-analysis effect size calculator 2016. http://www.campbellcollaboration.org/resources/effect_size_input.php, 2016.
EpiGear Internation Pty Ltd. (2015). Meta XL (5.0).
DerSimonian, R., & Laird, N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188.
Higgins, J. P., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560.
Sawilowsky, S. (2009). New effect size rules of thumb. Journal of Modern Applied Statistical Methods, 8(2), 597–599.
Kraemer, H. C., & Kupfer, D. J. (2006). Size of treatment effects and their importance to clinical research and practice. Biological Psychiatry, 59(11), 990–996.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences. New York: Academic Press.
Frich, L. M., Sorensen, J., Jacobsen, S., Fohlmann, B., & Hojsted, J. (2012). Outcomes of follow-up visits to chronic nonmalignant pain patients. Pain Management Nursing: Official Journal of The American Society of Pain Management Nurses, 13(4), 223–235.
Sacks, H. S., Chalmers, T. C., & Smith, H. (1982). Randomized versus historical controls for clinical trials. American Journal of Medicine, 72, 233–240.
Schulz, K. F., Chalmers, I., Hayes, R., & Altman, D. G. (1995). Empirical evidence of bias: Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association, 273, 408–412.
Funding
GAK was supported by the National Institutes of Health, National Institute of General Medical Sciences (Grant Number U54GM104942). JDT was supported by the National Institutes of Health, National Institute of General Medical Sciences (Grant Number 5T32GM081741-08). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JDT, RG, ND, and GAK declare that they have no conflicts of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thornton, J.D., Goyat, R., Dwibedi, N. et al. Health-related quality of life in patients receiving long-term opioid therapy: a systematic review with meta-analysis. Qual Life Res 26, 1955–1967 (2017). https://doi.org/10.1007/s11136-017-1538-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-017-1538-0