Abstract
Purpose
To assess the efficacy of the novel clinical formulation of fenretinide (LAU-7b) for the treatment of allergic asthma. To study the association between LAU-7b treatment in allergic asthma and the modulation of very long chain ceramides (VLCC).
Methods
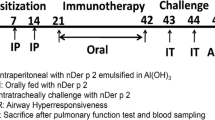
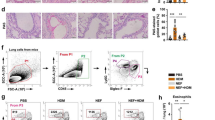
We used two allergens (OVA and HDM) to induce asthma in mouse models and we established a treatment protocol with LAU-7b. The severity of allergic asthma reaction was quantified by measuring the airway resistance, quantifying lung inflammatory cell infiltration (Haematoxylin and eosin stain) and mucus production (Periodic acid Schiff satin). IgE levels were measured by ELISA. Immunophenotyping of T cells was done using Fluorescence-activated cell sorting (FACS) analysis. The analysis of the specific species of lipids and markers of oxidation was performed using mass spectrometry.
Results
Our data demonstrate that 10 mg/kg of LAU-7b was able to protect OVA- and HDM-challenged mice against increase in airway hyperresponsiveness, influx of inflammatory cells into the airways, and mucus production without affecting IgE levels. Treatment with LAU-7b significantly increased percentage of regulatory T cells and CD4+ IL-10-producing T cells and significantly decreased percentage of CD4+ IL-4-producing T cells. Our data also demonstrate a strong association between the improvement in the lung physiology and histology parameters and the drug-induced normalization of the aberrant distribution of ceramides in allergic mice.
Conclusion
9 days of 10 mg/kg of LAU-7b daily treatment protects the mice against allergen-induced asthma and restores VLCC levels in the lungs and plasma.













Similar content being viewed by others
Abbreviations
- AA:
-
Arachidonic acid
- AHR:
-
Airway hyperresponsiveness
- ANOVA:
-
Analysis of variance
- C1P:
-
Ceramide-1-phosphate
- DHA:
-
Docosahexaenoic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- FEN:
-
Fenretinide
- H&E:
-
Haematoxylin and eosin
- HDM:
-
House dust mite
- i.n.:
-
Intranasal
- i.p.:
-
Intraperitoneal
- LCC:
-
Long chain ceramides
- MDA:
-
Malondialdehyde
- MCh:
-
Methacholine
- NT:
-
Nitrotyrosine
- OVA:
-
Ovalbumin
- p.o.:
-
per os
- PAS:
-
Periodic acid Schiff
- S1P:
-
Sphingosine-1-phosphate
- VLCC:
-
Very long chain ceramides
References
Park H-W, Tantisira KG, Weiss ST. Pharmacogenomics in asthma therapy: where are we and where do we go? Annu Rev Pharmacol Toxicol. 2015;55(1):129–47.
Youssef M, Kanagaratham C, Saad MI, Radzioch D. Genetics of allergic asthma and current perspectives on therapeutic management. In: Asthma - From Childhood Asthma to ACOS Phenotypes. 1st ed. InTech; 2016. p. 137–184.
Kaplan A. Asthma in adults. In: Barry Power, editor. Compendium of therapeutic choices. 2019 Editi. Canadian Pharmacists Association: Ottawa ON; 2019.
Sossai P, Travaglione AM, Amenta F. Asthma : Opinion or evidence based medicine ? 2014;4(2):17–22.
Guilbault C, De Sanctis JB, Wojewodka G, Saeed Z, Lachance C, Skinner TAA, et al. Fenretinide corrects newly found ceramide deficiency in cystic fibrosis. Am J Respir Cell Mol Biol. 2008;38(1):47–56.
Guilbault C, Wojewodka G, Saeed Z, Hajduch M, Matouk E, De Sanctis JB, et al. Cystic fibrosis fatty acid imbalance is linked to ceramide deficiency and corrected by fenretinide. Am J Respir Cell Mol Biol. 2009;41(1):100–6.
Kanagaratham C, Kalivodová A, Najdekr L, Friedecký D, Adam T, Hajduch M, et al. Fenretinide prevents inflammation and airway hyperresponsiveness in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2014;51(6):783–92.
López-Vales R, Redensek A, Skinner TAA, Rathore KI, Ghasemlou N, Wojewodka G, et al. Fenretinide promotes functional recovery and tissue protection after spinal cord contusion injury in mice. J Neurosci. 2010;30(9):3220–6.
Aun MV, Bonamichi-Santos R, Arantes-Costa FM, Kalil J, Giavina-Bianchi P. Animal models of asthma: utility and limitations. J Asthma Allergy. 2017;10(7):293–301.
Folch J, Lees M, Sloane SGH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509.
Garić D, Tao S, Ahmed E, Youssef M, Kanagaratham C, Shah J, et al. Depletion of BAFF cytokine exacerbates infection in Pseudomonas aeruginosa infected mice. J Cyst Fibros. 2019;18(3):349–56.
Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–25.
Sagar S, Akbarshahi H, Uller L. Translational value of animal models of asthma: challenges and promises. Eur J Pharmacol. 2015;759(15):272–7.
Bracken SJ, Adami AJ, Szczepanek SM, Ehsan M, Natarajan P, Guernsey LA, et al. Long-term exposure to house dust mite leads to the suppression of allergic airway disease despite persistent lung inflammation. Int Arch Allergy Immunol. 2015;166(4):243–58.
Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169(3):378–85.
Sabichi AL, Lerner SP, Atkinson EN, Grossman HB, Caraway NP, Dinney CP, Penson DF, Matin S, Kamat A, Pisters LL, Lin DW, Katz RL, Brenner DE, Hemstreet GP 3rd, Wargo M, Bleyer A, Sanders WH, Clifford JL, Parnes HL, Lippman SM Phase III prevention trial of fenretinide in patients with resected non-muscle-invasive bladder cancer. Clin Cancer Res 2008;14(1):224–229.
Colombo N, Formelli F, Cantù MG, Parma G, Gasco M, Argusti A, et al. A phase I-II preoperative biomarker trial of fenretinide in ascitic ovarian cancer. Cancer Epidemiol Biomark Prev. 2006;15(10):1914–9.
Moore MM, Stockler M, Lim R, Mok TSK, Millward M, Boyer MJ. A phase II study of fenretinide in patients with hormone refractory prostate cancer: a trial of the Cancer therapeutics research group. Cancer Chemother Pharmacol. 2010;66(5):845–50.
Nieto K, Pei P, Wang D, Mallery SR, Schwendeman SP. In vivo controlled release of fenretinide from long-acting release depots for chemoprevention of oral squamous cell carcinoma recurrence. Int J Pharm. 2018;538(1–2):48–56.
Mohrbacher AM, Yang AS, Groshen S, Kummar S, Gutierrez ME, Kang MH, et al. Phase I study of Fenretinide delivered intravenously in patients with relapsed or refractory hematologic malignancies: a California Cancer consortium trial. Clin Cancer Res. 2017;23(16):4550–5.
Cooper JP, Reynolds CP, Cho H, Kang MH. Clinical development of fenretinide as an antineoplastic drug: pharmacology perspectives. Exp Biol Med (Maywood). 2017;242(11):1178–84.
Maurer BJ, Kalous O, Yesair DW, Wu X, Janeba J, Maldonado V, et al. Improved oral delivery of N-(4-hydroxyphenyl)retinamide with a novel LYM-X-SORB organized lipid complex. Clin Cancer Res. 2007;13(10):3079–86.
Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MCJM, Verhamme KMC. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45(2):396–407.
Canals D, Salamone S, Hannun YA. Visualizing bioactive ceramides. Chem Phys Lipids. 2018;216(1):142–51.
Kiefer K, Casas J, García-López R, Vicente R. Ceramide Imbalance and Impaired TLR4-Mediated Autophagy in BMDM of an ORMDL3-Overexpressing Mouse Model. Int J Mol Sci. 2019;20(6):pii: E1391.
Zhang Y, Willis-Owen SAG, Spiegel S, Lloyd CM, Moffatt MF, Cookson WOCM. The ORMDL3 asthma gene regulates ICAM1 and has multiple effects on cellular inflammation. Am J Respir Crit Care Med. 2018;199(4):478–88.
Garić D, De Sanctis JB, Shah J, Dumut DC, Radzioch D. Biochemistry of very-long-chain and long-chain ceramides in cystic fibrosis and other diseases: the importance of side chain. Prog Lipid Res. 2019;74(1):130–44.
Scholte BJ, Horati H, Veltman M, Vreeken RJ, Garratt LW, Tiddens HAWM, et al. Oxidative stress and abnormal bioactive lipids in early cystic fibrosis lung disease. J Cyst Fibros. 2019;pii: S1569(19):30071–2.
Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51(1):50–62.
Garić D, De Sanctis JB, Wojewodka G, Houle D, Cupri S, Abu-Arish A, et al. Fenretinide differentially modulates the levels of long- and very long-chain ceramides by downregulating Cers5 enzyme: evidence from bench to bedside. J Mol Med (Berl). 2017;95(10):1053–64.
Kanagaratham C, Chiwara V, Ho B, Moussette S, Youssef M, Venuto D, et al. Loss of the zona pellucida-binding protein 2 (Zpbp2) gene in mice impacts airway hypersensitivity and lung lipid metabolism in a sex-dependent fashion. Mamm Genome. 2018;29(3–4):281–98.
Laviad EL, Kelly S, Merrill AH, Futerman AH. Modulation of ceramide synthase activity via dimerization. J Biol Chem. 2012;287(25):21025–33.
Chang K-T, Anishkin A, Patwardhan GA, Beverly LJ, Siskind LJ, Colombini M. Ceramide channels: destabilization by Bcl-xL and role in apoptosis. Biochim Biophys Acta. 2015;1848(10 Pt a):2374–84.
Stiban J, Perera M. Very long chain ceramides interfere with C16-ceramide-induced channel formation: A plausible mechanism for regulating the initiation of intrinsic apoptosis. Biochim Biophys Acta. 2015 Feb;1848(2):561–7.
Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100(8):1307–17.
Lougheed MD, Lemiere C, Ducharme FM, Licskai C, Dell SD, Rowe BH, et al. Canadian thoracic society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J. 2012;19(2):127–64.
Anderson SD. Repurposing drugs as inhaled therapies in asthma. Adv Drug Deliv Rev. 2018;133(1):19–33.
McManus R. Mechanisms of steroid action and resistance in inflammation and disease. J Endocrinol. 2003;178(1):1–4.
Adcock I, Lane S. Corticosteroid-insensitive asthma: molecular mechanisms. J Endocrinol. 2003;178(3):347–55.
SCHACKE H. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23–43.
Camerini T, Mariani L, De Palo G, Marubini E, Di Mauro MG, Decensi A, et al. Safety of the synthetic retinoid fenretinide: long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J Clin Oncol. 2001;19(6):1664–70.
Mata NL, Lichter JB, Vogel R, Han Y, Bui T V, Singerman LJ. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina. 2013;33(3):498–507.
Alzoghaibi MA, Walsh SW, Willey A, Yager DR, Fowler AA, Graham MF. Linoleic acid induces interleukin-8 production by Crohn’s human intestinal smooth muscle cells via arachidonic acid metabolites. Am J Physiol Gastrointest Liver Physiol. 2004;286(4):G528–37.
Vilela RM, Lands LC, Meehan B, Kubow S. Inhibition of IL-8 release from CFTR-deficient lung epithelial cells following pre-treatment with fenretinide. Int Immunopharmacol. 2006;6(11):1651–64.
Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402(6760 Suppl):B18–23.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Materials
ESM 1
(DOCX 1659 kb)
Rights and permissions
About this article
Cite this article
Youssef, M., De Sanctis, J.B., Kanagaratham, C. et al. Efficacy of Optimized Treatment Protocol Using LAU-7b Formulation against Ovalbumin (OVA) and House Dust Mite (HDM) -Induced Allergic Asthma in Atopic Hyperresponsive A/J Mice. Pharm Res 37, 31 (2020). https://doi.org/10.1007/s11095-019-2743-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2743-z