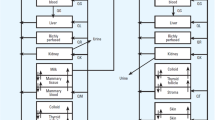
Abstract
One impediment to breastfeeding is the lack of information on the use of many drugs during lactation, especially newer ones. The principles of drug passage into breastmilk are well established, but have often not been optimally applied prospectively. Commonly used preclinical rodent models for determining drug excretion into milk are very unreliable because of marked differences in milk composition and transporters compared to those of humans. Measurement of drug concentrations in humans remains the gold standard, but computer modeling is promising. New FDA labeling requirements present an opportunity to apply modeling to preclinical drug development in place of conventional animal testing for drug excretion into breastmilk, which should improve the use of medications in nursing mothers.



Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the concentration-time curve
- CL:
-
Total body drug clearance
- Cp:
-
Drug concentration in plasma
- CYP2D6:
-
Cytochrome P450 2D6
- F:
-
Bioavailability
- FDA:
-
U.S. Food and Drug Administration
- HIV:
-
Human immunodeficiency virus
- I/M:
-
Infant to maternal plasma concentration ratio
- M/P:
-
Milk to plasma concentration ratio
- PBPK:
-
Physiologically based pharmacokinetic
- PLLR:
-
Pregnancy and Lactation Labeling Rule
- PopPK:
-
Population pharmacokinetics
- QSAR:
-
Quantitative structure-activity relationship
- RID:
-
Relative infant dose
- WHO:
-
World Health Organization
References
AAP Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–41.
Bartick MC, Schwarz EB, Green BD, Jegier BJ, Reinhold AG, Colaizy TT, et al. Suboptimal breastfeeding in the United States: maternal and pediatric health outcomes and costs. Matern Child Nutr. 2017;13:e12366.
World Health Organization. Global strategy for infant and young child feeding. 2003. Geneva. http://www.unicef.org/nutrition/files/Global_Strategy_Infant_and_Young_Child_Feeding.pdf. Accessed 2 Aug 2017.
Centers for Disease Control and Prevention. Breastfeeding Report Card, 2016. https://www.cdc.gov/breastfeeding/data/reportcard.htm. Accessed 6 July 2017.
Ito S, Lieu M, Chan W, Koren G. Continuing drug therapy while breastfeeding. Part 1. Common misconceptions of patients. Can Fam Physician. 1999;45:897–9.
Acevedo M, Pretini J, Micelli M, Sequeira G, Kerzberg E. Breastfeeding initiation, duration, and reasons for weaning in patients with systemic lupus erythematosus. Rheumatol Int. 2017;37:1183–6.
Ohman I, Vitols S, Tomson T. Lamotrigine in pregnancy: pharmacokinetics during delivery, in the neonate, and during lactation. Epilepsia. 2000;41:709–13.
Giorda G, de Vincentiis S, Motta T, Casazza S, Fadin M, D’Alberton A. Cabergoline versus bromocriptine in suppression of lactation after cesarean delivery. Gynecol Obstet Investig. 1991;31:93–6.
Bernard N, Jantzem H, Becker M, Pecriaux C, Benard-Laribiere A, Montastruc JL, et al. Severe adverse effects of bromocriptine in lactation inhibition: a pharmacovigilance survey. BJOG. 2015;122:1244–51.
Aljazaf K, Hale TW, Ilett KF, Hartmann PE, Mitoulas LR, Kristensen JH, et al. Pseudoephedrine: effects on milk production in women and estimation of infant exposure via breastmilk. Br J Clin Pharmacol. 2003;56:18–24.
Svennersten K, Nelson L, Juvnas-Moberg K. Atropinization decreases oxytocin secretion in dairy cows. Acta Physiol Scand. 1992;145:193–4.
Daniel JA, Thomas MG, Powell MR, Keisler DH. Methscopolamine bromide blocks hypothalmic-stimulated release of growth hormone in ewes. J Anim Sci. 1997;75:1359–62.
Masala A, Alagna S, Devilla L, Rovasio PP, Rassa S, Faedda R, et al. Muscarinic receptor blockade by pirenzepine: effect on prolactin secretion in man. J Endocrinol Investig. 1982;5:53–5.
Messinis IE, Souvatzoglou A, Fais N, Lolis D. Histamine H1 receptor participation in the control of prolactin secretion in postpartum. J Endocrinol Investig. 1985;8:143–6.
World Health Organization. Medical eligibility criteria for contraceptive use: fifth ed. 2015; http://www.who.int/reproductivehealth/publications/family_planning/MEC-5/en/.
Donovan TJ, Buchanan K. Medications for increasing milk supply in mothers expressing breastmilk for their preterm hospitalised infants. Cochrane Database Syst Rev. 2012;3:CD005544.
Asztalos EV, Campbell-Yeo M, da Silva OP, Ito S, Kiss A, Knoppert D. Enhancing human milk production with domperidone in mothers of preterm infants. J Hum Lact. 2017;33:181–7.
Anderson PO. Domperidone: the forbidden fruit. Breastfeed Med. 2017;12:258–60.
Rasmussen F. Excretion of drugs by milk. In: Brodie BB, Gillette JR, editors. Concepts in biochemical pharmacology, part 1. New York: Springer-Verlag; 1971. p. 390–402.
Rasmussen F. The mechanism of drug secretion into milk. In: Galli C, Jacini G, Pecile A, editors. Dietary lipids and postnatal development. New York: Raven Press; 1973. p. 231–45.
Matheson I, Samseth M, Loberg R, Faegri A, Prentice A. Milk transfer of phenoxymethylpenicillin during puerperal mastitis. Br J Clin Pharmacol. 1988;25:33–40.
Hunt KM, Williams JE, Shafii B, Hunt MK, Behre R, Ting R, et al. Mastitis is associated with increased free fatty acids, somatic cell count, and interleukin-8 concentrations in human milk. Breastfeed Med. 2013;8:105–10.
Tuaillon E, Viljoen J, Dujols P, Cambonie G, Rubbo PA, Nagot N, et al. Subclinical mastitis occurs frequently in association with dramatic changes in inflammatory/anti-inflammatory breast milk components. Pediatr Res. 2017;81:556–64.
Atkinson HC, Begg EJ. Prediction of drug distribution into human milk from physicochemical characteristics. Clin Pharmacokinet. 1990;18:151–67.
Ansell C, Moore A, Barrie H. Electrolyte and pH changes in human milk. Pediatr Res. 1977;11:1177–9.
Fleishaker JC, Desai N, McNamara PJ. Factors affecting the milk-to-plasma drug concentration ratio in lactating women: physical interactions with protein and fat. J Pharm Sci. 1987;76:189–93.
Atkinson HC, Begg EJ. The binding of drugs to major human milk whey proteins. Br J Clin Pharmacol. 1988;26:107–9.
Anderson GD. Using pharmacokinetics to predict the effects of pregnancy and maternal-infant transfer of drugs during lactation. Expert Opin Drug Metab Toxicol. 2006;2:947–60.
Koletzko B. Human milk lipids. Ann Nutr Metab. 2016;69(Suppl 2):28–40.
Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117:e387–95.
Mangel L, Ovental A, Batscha N, Arnon M, Yarkoni I, Dollberg S. Higher fat content in breastmilk expressed manually: a randomized trial. Breastfeed Med. 2015;10:352–4.
Syversen GB, Ratkje SK. Drug distribution within human milk phases. J Pharm Sci. 1985;74:1071–4.
Atkinson HC, Begg EJ. Relationship between human milk lipid-ultrafiltrate and octanol-water partition coefficients. J Pharm Sci. 1988;77:796–8.
Ito S, Alcorn J. Xenobiotic transporter expression and function in the human mammary gland. Adv Drug Deliv Rev. 2003;55:653–65.
Darrouzet E, Lindenthal S, Marcellin D, Pellequer JL, Pourcher T. The sodium/iodide symporter: state of the art of its molecular characterization. Biochim Biophys Acta. 2014;1838(1 Pt. B):244–53.
Ito N, Ito K, Ikebuchi Y, Toyoda Y, Takada T, Hisaka A, et al. Prediction of drug transfer into milk considering breast cancer resistance protein (BCRP)-mediated transport. Pharm Res. 2015;32:2527–37.
Delaney S, Ito S, Colantonio D. Drug-monitoring of methotrexate in breast milk: understanding transport from cells to humans. Clin Biochem. 2016;49:Abstract P136.
Kwok B, Yamauchi A, Rajesan R, Chan L, Dhillon U, Gao W, et al. Carnitine/xenobiotics transporters in the human mammary gland epithelia, MCF12A. Am J Phys Regul Integr Comp Phys. 2006;290:R793–802.
Kimura S, Morimoto K, Okamoto H, Ueda H, Kobayashi D, Kobayashi J, et al. Development of a human mammary epithelial cell culture model for evaluation of drug transfer into milk. Arch Pharm Res. 2006;29:424–9.
Quezada A, Vafai K. Modeling and analysis of transport in the mammary glands. Phys Biol. 2014;11:045004.
da-Silva VA, Malheiros LR, Moraes-Santos AR, Barzano MA, AE ML. Ethanol pharmacokinetics in lactating women. Braz J Med Biol Res. 1993;26:1097–103.
Oo CY, Kuhn RJ, Desai N, Wright CE, McNamara PJ. Pharmacokinetics in lactating women: prediction of alprazolam transfer into milk. Br J Clin Pharmacol. 1995;40:231–6.
Nouws JF, Ziv G. Pharmacological aspects of chloramphenicol administration by intramammary route to lactating dairy cows. Vet Q. 1982;4:23–31.
Erskine RJ, Wilson RC, Riddell MG Jr, Tyler JW, Spears HJ, Davis BS. Intramammary administration of gentamicin as treatment for experimentally induced Escherichia coli mastitis in cows. Am J Vet Res. 1992;53:375–81.
Garrett EF, Dirikolu L, Grover GS. Milk and serum concentration of ceftiofur following intramammary infusion in goats. J Vet Pharmacol Ther. 2015;38:569–74.
Koshimichi H, Ito K, Honma M, Hisaka A, Suzuki H. Analysis and prediction of drug transfer into human milk taking into consideration secretion and reuptake clearances across the mammary epithelia. Drug Metab Dispos. 2011;39:2370–80.
Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55:667–86.
Matalova P, Urbanek K, Anzenbacher P. Specific features of pharmacokinetics in children. Drug Metab Rev. 2016;48:70–9.
Axelsson I, Jakobsson I, Lindberg T, Polberger S, Benediktsson B, Raiha N. Macromolecular absorption in preterm and term infants. Acta Paediatr Scand. 1989;78:532–7.
Kuitunen M, Savilahti E, Sarnesto A. Human alpha-lactalbumin and bovine beta-lactoglobulin absorption in infants. Allergy. 1994;49:354–60.
Saleem B, Okogbule-Wonodi AC, Fasano A, Magder LS, Ravel J, Kapoor S, et al. Intestinal barrier maturation in very low birthweight infants: relationship to feeding and antibiotic exposure. J Pediatr. 2017;183:31–6.e1.
Taylor SN, Basile LA, Ebeling M, Wagner CL. Intestinal permeability in preterm infants by feeding type: mother’s milk versus formula. Breastfeed Med. 2009;4:11–5.
Mooij MG, Schwarz UI, de Koning BA, Leeder JS, Gaedigk R, Samsom JN, et al. Ontogeny of human hepatic and intestinal transporter gene expression during childhood: age matters. Drug Metab Dispos. 2014;42:1268–74.
Verner MA, Plouffe L, Kieskamp KK, Rodriguez-Leal I, Marchitti SA. Evaluating the influence of half-life, milk: plasma partition coefficient, and volume of distribution on lactational exposure to chemicals in children. Environ Int. 2017;102:223–9.
Berle JO, Steen VM, Aamo TO, Breilid H, Zahlsen K, Spigset O. Breastfeeding during maternal antidepressant treatment with serotonin reuptake inhibitors: infant exposure, clinical symptoms, and cytochrome P450 genotypes. J Clin Psychiatry. 2004;65:1228–34.
Madadi P, Ross C, Hayden M, Carleton B, Gaedigk A, Leeder J, et al. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case-control study. Clin Pharmacol Ther. 2009;85:31–5.
Olagunju A, Owen A, Cressey TR. Potential effect of pharmacogenetics on maternal, fetal and infant antiretroviral drug exposure during pregnancy and breastfeeding. Pharmacogenomics. 2012;13:1501–22.
Soussan C, Gouraud A, Portolan G, Jean-Pastor MJ, Pecriaux C, Montastruc JL, et al. Drug-induced adverse reactions via breastfeeding: a descriptive study in the French Pharmacovigilance Database. Eur J Clin Pharmacol. 2014;70:1361–6.
Anderson PO, Manoguerra AS, Valdes V. A review of adverse reactions in infants from medications in breastmilk. Clin Pediatr (Phila). 2016;55:236–44.
Ito N, Ito K, Koshimichi H, Hisaka A, Honma M, Igarashi T, et al. Contribution of protein binding, lipid partitioning, and asymmetrical transport to drug transfer into milk in mouse versus human. Pharm Res. 2013;30:2410–22.
Wang J, Johnson T, Sahin L, Tassinari MS, Anderson PO, Baker TE, et al. Evaluation of the safety of drugs and biological products used during lactation: workshop summary. Clin Pharmacol Ther. 2017;101:736–44.
Nicholas KR, Hartmann PE. Milk secretion in the rat: progressive changes in milk composition during lactation and weaning and the effect of diet. Comp Biochem Physiol A Comp Physiol. 1991;98:535–42.
Godbole VY, Grundleger ML, Pasquine TA, Thenen SW. Composition of rat milk from day 5 to 20 of lactation and milk intake of lean and preobese Zucker pups. J Nutr. 1981;111:480–7.
Begg EJ, Duffull SB, Hackett LP, Ilett KF. Studying drugs in milk: time to unify the approach. J Hum Lact. 2002;18:323–32.
Food and Drug Administration. Guidance for industry. Clinical lactation studies -- study design, data analysis and recommendations for labeling. Draft guidance. 2005; https://www.fda.gov/RegulatoryInformation/Guidances/ucm127484.htm. Accessed 31 July 2017.
Somogyi A, Gugler R. Cimetidine excretion into breast milk. Br J Clin Pharmacol. 1979;7:627–9. Letter
Bennett PN. Drugs and human lactation, 1st ed. Amsterdam: Elsevier; 1988. p. 29.
Neville MC, Keller R, Seacat J, Lutes V, Neifert M, Casey C, et al. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988;48:1375–86.
Anderson PO, Valdes V. Variation of milk intake over time: clinical and pharmacokinetic implications. Breastfeed Med. 2015;10:142–4.
Atkinson HC, Begg EJ, Darlow BA. Drugs in human milk: clinical pharmacokinetic considerations. Clin Pharmacokinet. 1988;14:217–40.
Rowe HE, Felkins K, Cooper SD, Hale TW. Transfer of linezolid into breast milk. J Hum Lact. 2014;30:410–2.
Wilson JT, Brown RD, Hinson JL, Dailey JW. Pharmacokinetic pitfalls in the estimation of the breast milk/plasma ratio for drugs. Annu Rev Pharmacol Toxicol. 1985;25:667–89.
Leroux S, Turner MA, Guellec CB, Hill H, van den Anker JN, Kearns GL, et al. Pharmacokinetic studies in neonates: the utility of an opportunistic sampling design. Clin Pharmacokinet. 2015;54:1273–85.
Meador KJ, Baker GA, Browning N, Cohen MJ, Bromley RL, Clayton-Smith J, et al. Breastfeeding in children of women taking antiepileptic drugs: cognitive outcomes at age 6 years. JAMA Pediatr. 2014;168:729–36.
Doogue MP, Gardiner SJ, Begg EJ. Prediction of milk/plasma concentration ratio of drugs. Ann Pharmacother. 2004;38:174–6.
Korzekwa K, Nagar S. On the nature of physiologically-based pharmacokinetic models -- a priori or a posteriori? Mechanistic or empirical? Pharm Res. 2017;34:529–34.
Kar S, Roy K. Prediction of milk/plasma concentration ratios of drugs and environmental pollutants using in silico tools: classification and regression based QSARs and pharmacophore mapping. Mol Inf. 2013;32:693–705.
Vasios G, Kosmidi A, Kalantzi OI, Tsantili-Kakoulidou A, Kavantzas N, Theocharis S, et al. Simple physicochemical properties related with lipophilicity, polarity, molecular size and ionization status exert significant impact on the transfer of drugs and chemicals into human breast milk. Expert Opin Drug Metab Toxicol. 2016;12:1273–8.
Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42–52.
Ilett KF, Paech MJ, Page-Sharp M, Sy SK, Kristensen JH, Goy R, et al. Use of a sparse sampling study design to assess transfer of tramadol and its O-desmethyl metabolite into transitional breast milk. Br J Clin Pharmacol. 2008;65:661–6.
Salman S, Sy SK, Ilett KF, Page-Sharp M, Paech MJ. Population pharmacokinetic modeling of tramadol and its O-desmethyl metabolite in plasma and breast milk. Eur J Clin Pharmacol. 2011;67:899–908.
Panchaud A, Garcia-Bournissen F, Csajka C, Kristensen JH, Taddio A, Ilett KF, et al. Prediction of infant drug exposure through breastfeeding: population PK modeling and simulation of fluoxetine exposure. Clin Pharmacol Ther. 2011;89:830–6.
Tanoshima R, Bournissen FG, Tanigawara Y, Kristensen JH, Taddio A, Ilett KF, et al. Population PK modelling and simulation based on fluoxetine and norfluoxetine concentrations in milk: a milk concentration-based prediction model. Br J Clin Pharmacol. 2014;78:918–28.
Paech MJ, Salman S, Ilett KF, O'Halloran SJ, Muchatuta NA. Transfer of parecoxib and its primary active metabolite valdecoxib via transitional breastmilk following intravenous parecoxib use after cesarean delivery: a comparison of naive pooled data analysis and nonlinear mixed-effects modeling. Anesth Analg. 2012;114:837–44.
Kunz A, Frank M, Mugenyi K, Kabasinguzi R, Weidenhammer A, Kurowski M, et al. Persistence of nevirapine in breast milk and plasma of mothers and their children after single-dose administration. J Antimicrob Chemother. 2009;63:170–7.
Garcia-Bournissen F, Altcheh J, Panchaud A, Ito S. Is use of nifurtimox for the treatment of Chagas disease compatible with breastfeeding? A population pharmacokinetics analysis. Arch Dis Child. 2010;95:224–8.
Moore BR, Salman S, Benjamin J, Page-Sharp M, Yadi G, Batty KT, et al. Pharmacokinetics of piperaquine transfer into the breast milk of Melanesian mothers. Antimicrob Agents Chemother. 2015;59:4272–8.
Salman S, Davis TM, Page-Sharp M, Camara B, Oluwalana C, Bojang A, et al. Pharmacokinetics of transfer of azithromycin into the breast milk of African mothers. Antimicrob Agents Chemother. 2016;60:1592–9.
Anderson PO, Pochop SL, Manoguerra AS. Adverse drug reactions in breastfed infants: less than imagined. Clin Pediatr (Phila). 2003;42:325–40.
Frank M, Harms G, Kunz A, Kloft C. Population pharmacokinetic analysis of a nevirapine-based HIV-1 prevention of mother-to-child transmission program in Uganda to assess the impact of different dosing regimens for newborns. J Clin Pharmacol. 2013;53:294–304.
Leong R, Vieira ML, Zhao P, Mulugeta Y, Lee CS, Huang SM, et al. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin Pharmacol Ther. 2012;91:926–31.
Luzon E, Blake K, Cole S, Nordmark A, Versantvoort C, Berglund EG. Physiologically based pharmacokinetic modeling in regulatory decision-making at the European medicines agency. Clin Pharmacol Ther. 2017;102:98–105.
Clewell RA, Gearhart JM. Pharmacokinetics of toxic chemicals in breast milk: use of PBPK models to predict infant exposure. Environ Health Perspect. 2002;110:A333–7.
Corley RA, Mast TJ, Carney EW, Rogers JM, Daston GP. Evaluation of physiologically based models of pregnancy and lactation for their application in children’s health risk assessments. Crit Rev Toxicol. 2003;33:137–211.
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45:1013–34.
Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J. Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clin Pharmacol Ther. 2009;86:634–43.
Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet. 2006;368:704.
Cibert M, Gouraud A, Vial T, Tod M. A physiologically-based pharmacokinetic model to predict neonate exposure to drugs during breast-feeding: application to lamotrigine. Fundam Clin Pharmacol. 2010;24(Suppl. 1):51. Abstract 246
Guedat MG, Gouraud A, Vial T, Tod M. A physiologically-based pharmacokinetic model for predicting neonate dose after breast-feeding by women treated with clonidine. Int J Clin Pharm. 2011;33:318. Abstract
Jorga K, Chavanne C, Frey N, Lave T, Lukacova V, Parrott N, et al. Bottom-up meets top-down: complementary physiologically based pharmacokinetic and population pharmacokinetic modeling for regulatory approval of a dosing algorithm of valganciclovir in very young children. Clin Pharmacol Ther. 2016;100:761–9.
Delaney S, Malik P, Takeuchi M, Wong E, Stefan C, Edginton A, et al. Drug monitoring and physiologically-based pharmacokinetic modeling of escitalopram in breast milk: predicting plasma exposure in breast-feeding infants. Birth Defects Res. 2017;109(Special Issue: SI):704. Abstract
Food and Drug Administration. Pregnancy and lactation labeling (Drugs) final rule. 2014; https://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed 30 June 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author thanks Edmund Capparelli, Pharm. D. for his review of the manuscript and helpful suggestions. The author declares no competing financial interests.
Additional information
Guest Editor: Sara Eyal
Rights and permissions
About this article
Cite this article
Anderson, P.O. Drugs in Lactation. Pharm Res 35, 45 (2018). https://doi.org/10.1007/s11095-017-2287-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-017-2287-z