Abstract
Purpose
The aim of this study is to evaluate the effects of diallyl sulfide (DAS) on the warm hepatic ischemia–reperfusion (IR) injury in a rat model.
Methods
Rats (n = 8–10/group) were subjected to sham operation or warm ischemia (1 h)-reperfusion (3 h) preceded by a single intraperitoneal dose (1.75 mmol/kg) of DAS or vehicle, and relevant biochemical parameters were monitored.
Results
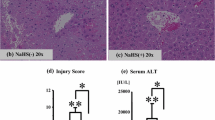
Warm IR injury caused a significant increase in the plasma markers of liver injury, which was attenuated by DAS. The hepatoprotective effects of DAS were associated with significant reductions in lipid peroxidation markers and in situ generation of superoxide in the liver and increases in the glutathione levels of the liver and bile, suggestive of an antioxidant effect for DAS. Additionally, DAS caused an almost twofold increase in the protein expression of the liver heme oxygenase-1, an enzyme that confers cytoprotection against oxidative stress. Whereas the total cytochrome P450 remained unchanged, the protein levels and activity of CYP2E1, which plays an important role in the generation of reactive oxygen species, significantly decreased by DAS pretreatment.
Conclusions
DAS protects the liver from warm IR injury by reducing oxidative stress through, at least in part, induction of heme oxygenase-1 and inhibition of CYP2E1.








Similar content being viewed by others
References
G. Martinez-Mier, L. H. Toledo-Pereyra, and P. A. Ward. Adhesion molecules in liver ischemia and reperfusion. J. Surg. Res. 94:185–194 (2000).
H. Jaeschke, and J. J. Lemasters. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 125:1246–1257 (2003).
S. N. Lichtman, and J. J. Lemasters. Role of cytokines and cytokine-producing cells in reperfusion injury to the liver. Semin. Liver Dis. 19:171–187 (1999).
H. Jaeschke. Role of reactive oxygen species in hepatic ischemia–reperfusion injury and preconditioning. J. Invest. Surg. 16:127–140 (2003).
J. W. Kupiec-Weglinski, and R. W. Busuttil. Ischemia and reperfusion injury in liver transplantation. Transplant Proc. 37:1653–1656 (2005).
H. Jaeschke. Molecular mechanisms of hepatic ischemia–reperfusion injury and preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 284:G15–G26 (2003).
F. Serracino-Inglott, N. A. Habib, and R. T. Mathie. Hepatic ischemia–reperfusion injury. Am. J. Surg. 181:160–166 (2001).
A. Casillas-Ramirez, I. B. Mosbah, F. Ramalho, J. Rosello-Catafau, and C. Peralta. Past and future approaches to ischemia–reperfusion lesion associated with liver transplantation. Life Sci. 79:1881–1894 (2006).
N. Selzner, H. Rudiger, R. Graf, and P. A. Clavien. Protective strategies against ischemic injury of the liver. Gastroenterology. 125:917–936 (2003).
C. Borek. Antioxidant health effects of aged garlic extract. J Nutr. 131:1010S–1015S (2001).
J. Imai, N. Ide, S. Nagae, T. Moriguchi, H. Matsuura, and Y. Itakura. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Medica. 60:417–420 (1994).
K. Prasad, V. A. Laxdal, M. Yu, and B. L. Raney. Evaluation of hydroxyl radical-scavenging property of garlic. Mol. Cell Biochem. 154:55–63 (1996).
H. P. Chang, and Y. H. Chen. Differential effects of organosulfur compounds from garlic oil on nitric oxide and prostaglandin E2 in stimulated macrophages. Nutrition. 21:530–536 (2005).
H. P. Chang, S. Y. Huang, and Y. H. Chen. Modulation of cytokine secretion by garlic oil derivatives is associated with suppressed nitric oxide production in stimulated macrophages. J. Agric. Food Chem. 53:2530–2534 (2005).
C. C. Wu, L. Y. Sheen, H. W. Chen, W. W. Kuo, S. J. Tsai, and C. K. Lii. Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J. Agric. Food Chem. 50:378–383 (2002).
D. Taubert, R. Glockner, D. Muller, and E. Schomig. The garlic ingredient diallyl sulfide inhibits cytochrome P450 2E1 dependent bioactivation of acrylamide to glycidamide. Toxicol Lett. 164:1–5 (2006).
P. Thejass, and G. Kuttan. Antiangiogenic activity of diallyl sulfide (DAS). Int. Immunopharmacol. 7:295–305 (2007).
J. F. Brady, M. H. Wang, J. Y. Hong, F. Xiao, Y. Li, J. S. Yoo, S. M. Ning, M. J. Lee, J. M. Fukuto, J. M. Gapac et al. Modulation of rat hepatic microsomal monooxygenase enzymes and cytotoxicity by diallyl sulfide. Toxicol. Appl. Pharmacol. 108:342–354 (1991).
J. J. Hu, J. S. Yoo, M. Lin, E. J. Wang, and C. S. Yang. Protective effects of diallyl sulfide on acetaminophen-induced toxicities. Food Chem. Toxicol. 34:963–969 (1996).
S. L. Fanelli, G. D. Castro, E. G. de Toranzo, and J. A. Castro. Mechanisms of the preventive properties of some garlic components in the carbon tetrachloride-promoted oxidative stress. Diallyl sulfide; diallyl disulfide; allyl mercaptan and allyl methyl sulfide. Res. Commun. Mol. Pathol. Pharmacol. 102:163–174 (1998).
P. Gong, B. Hu, and A. I. Cederbaum. Diallyl sulfide induces heme oxygenase-1 through MAPK pathway. Arch. Biochem. Biophys. 432:252–260 (2004).
L. Y. Sheen, C. K. Lii, S. F. Sheu, R. H. Meng, and S. J. Tsai. Effect of the active principle of garlic–diallyl sulfide-on cell viability, detoxification capability and the antioxidation system of primary rat hepatocytes. Food Chem. Toxicol. 34:971–978 (1996).
J. F. Brady, H. Ishizaki, J. M. Fukuto, M. C. Lin, A. Fadel, J. M. Gapac, and C. S. Yang. Inhibition of cytochrome P-450 2E1 by diallyl sulfide and its metabolites. Chem. Res. Toxicol. 4:642–647 (1991).
A. T. Canada, K. Stein, D. Martel, and W. D. Watkins. Biochemical appraisal of models for hepatic ischemia-reperfusion injury. Circ. Shock. 36:163–168 (1992).
H. U. Spiegel, and R. Bahde. Experimental models of temporary normothermic liver ischemia. J. Invest Surg. 19:113–123 (2006).
I. H. Shaik, and R. Mehvar. Rapid determination of reduced and oxidized glutathione levels using a new thiol-masking reagent and the enzymatic recycling method: application to the rat liver and bile samples. Anal. Bioanal. Chem. 385:105–113 (2006).
H. Ukeda, S. Maeda, T. Ishii, and M. Sawamura. Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3¢-1-(phenylamino)-carbonyl-3, 4-tetrazolium]-bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate reduction by xanthine-xanthine oxidase. Anal. Biochem. 251:206–209 (1997).
L. H. Johansson, and L. A. Borg. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 174:331–336 (1988).
R. Mateos, E. Lecumberri, S. Ramos, L. Goya, and L. Bravo. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 827:76–82 (2005).
V. Tong, T. K. Chang, J. Chen, and F. S. Abbott. The effect of valproic acid on hepatic and plasma levels of 15-F2t-isoprostane in rats. Free Radic. Biol. Med. 34:1435–1446 (2003).
B. G. Lake. Preparation and characterisation of microsomal fractions for studies on xenobiotic metabolism. In K. Snelland, and B. Mullock (eds.), Biochemical Toxicology, a Practical Approach, IRL, Oxford, UK, 1987, pp. 183–215.
R. Vuppugalla, and R. Mehvar. Hepatic disposition and effects of nitric oxide donors: rapid and concentration-dependent reduction in the cytochrome P450-mediated drug metabolism in isolated perfused rat livers. J. Pharmacol. Exp. Ther. 310:718–727 (2004).
T. Omura, and R. Sato. The carbon monoxide-binding pigment of the liver microsomes. Evidence for its hemoprotein nature. J. Biol. Chem. 239:2370–2378 (1964).
R. Vuppugalla, R. B. Shah, A. P. Chimalakonda, C. W. Fisher, and R. Mehvar. Microsomal cytochrome P450 levels and activities of isolated rat livers perfused with albumin. Pharm. Res. 20:81–88 (2003).
Y. Minamiyama, S. Takemura, S. Toyokuni, S. Imaoka, Y. Funae, K. Hirohashi, T. Yoshikawa, and S. Okada. CYP3A induction aggravates endotoxemic liver injury via reactive oxygen species in male rats. Free Radic. Biol. Med. 37:703–712 (2004).
J. Belghiti, K. Hiramatsu, S. Benoist, P. Massault, A. Sauvanet, and O. Farges. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J. Am. Coll. Surg. 191:38–46 (2000).
S. W. Ryter, J. Alam, and A. M. Choi. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 86:583–650 (2006).
H. Xue, H. Guo, Y. C. Li, and Z. M. Hao. Heme oxygenase-1 induction by hemin protects liver cells from ischemia/reperfusion injury in cirrhotic rats. World J. Gastroenterol. 13:5384–5390 (2007).
R. Schmidt, E. Tritschler, A. Hoetzel, T. Loop, M. Humar, L. Halverscheid, K. K. Geiger, and B. H. Pannen. Heme oxygenase-1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/reperfusion injury. Ann. Surg. 245:931–942 (2007).
X. D. Shen, B. Ke, Y. Zhai, F. Gao, R. W. Busuttil, G. Cheng, and J. W. Kupiec-Weglinski. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am. J. Transplant. 5:1793–1800 (2005).
H. Kato, F. Amersi, R. Buelow, J. Melinek, A. J. Coito, B. Ke, R. W. Busuttil, and J. W. Kupiec-Weglinski. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am. J. Transplant. 1:121–128 (2001).
H. Su, G. M. van Dam, C. I. Buis, D. S. Visser, J. W. Hesselink, T. A. Schuurs, H. G. Leuvenink, C. H. Contag, and R. J. Porte. Spatiotemporal expression of heme oxygenase-1 detected by in vivo bioluminescence after hepatic ischemia in HO-1/Luc mice. Liver Transpl. 12:1634–1639 (2006).
K. Ito, S. Ikeda, T. Shibata, T. Yano, and S. Horikawa. Immunohistochemical analysis of heme oxygenase-I in rat liver after ischemia. Biochem. Mol. Biol. Int. 43:551–556 (1997).
C. Chen, D. Pung, V. Leong, V. Hebbar, G. Shen, S. Nair, W. Li, and A. N. Kong. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic. Biol. Med. 37:1578–1590 (2004).
C. S. Yang, S. K. Chhabra, J. Y. Hong, and T. J. Smith. Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. J. Nutr. 131:1041S–1045S (2001).
A. Zhukov, and M. Ingelman-Sundberg. Relationship between cytochrome P450 catalytic cycling and stability: fast degradation of ethanol-inducible cytochrome P450 2E1 (CYP2E1) in hepatoma cells is abolished by inactivation of its electron donor NADPH-cytochrome P450 reductase. Biochem. J. 340(Pt 2):453–458 (1999).
R. C. Zangar, D. R. Davydov, and S. Verma. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol. Appl. Pharmacol. 199:316–331 (2004).
V. G. Lee, M. L. Johnson, J. Baust, V. E. Laubach, S. C. Watkins, and T. R. Billiar. The roles of iNOS in liver ischemia-reperfusion injury. Shock. 16:355–360 (2001).
I. N. Hines, H. Harada, S. Flores, B. Gao, J. M. McCord, and M. B. Grisham. Endothelial nitric oxide synthase protects the post-ischemic liver: potential interactions with superoxide. Biomed. Pharmacother. 59:183–189 (2005).
J. S. Beckman, and W. H. Koppenol. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 271:C1424–C1437 (1996).
H. Ohmori, D. K. Dhar, Y. Nakashima, M. Hashimoto, S. Masumura, and N. Nagasue. Beneficial effects of FK409, a novel nitric oxide donor, on reperfusion injury of rat liver. Transplantation. 66:579–585 (1998).
F. Langle, E. Roth, R. Steininger, S. Winkler, and F. Muhlbacher. Arginase release following liver reperfusion. Evidence of hemodynamic action of arginase infusions. Transplantation. 59:1542–1549 (1995).
L. Chen, J. Y. Hong, E. So, A. H. Hussin, W. F. Cheng, and C. S. Yang. Decrease of hepatic catalase level by treatment with diallyl sulfide and garlic homogenates in rats and mice. J. Biochem. Mol. Toxicol. 13:127–134 (1999).
E. Niki, Y. Yoshida, Y. Saito, and N. Noguchi. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 338:668–676 (2005).
M. Fukai, T. Hayashi, R. Yokota, T. Shimamura, T. Suzuki, M. Taniguchi, M. Matsushita, H. Furukawa, and S. Todo. Lipid peroxidation during ischemia depends on ischemia time in warm ischemia and reperfusion of rat liver. Free Radic. Biol. Med. 38:1372–1381 (2005).
D. R. Janero. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 9:515–540 (1990).
G. Sener, O. Sehirli, Y. Ipci, F. Ercan, S. Sirvanci, N. Gedik, and B. C. Yegen. Aqueous garlic extract alleviates ischaemia–reperfusion-induced oxidative hepatic injury in rats. J. Pharm. Pharmacol. 57:145–150 (2005).
Acknowledgement
The authors would like to thank Dr. Paul R. Lockman for assistance with fluorescence microscopy and Dr. Donald L. Montgomery from the University of Wyoming for histological studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaik, I.H., George, J.M., Thekkumkara, T.J. et al. Protective Effects of Diallyl Sulfide, a Garlic Constituent, on the Warm Hepatic Ischemia–Reperfusion Injury in a Rat Model. Pharm Res 25, 2231–2242 (2008). https://doi.org/10.1007/s11095-008-9601-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9601-8