Abstract
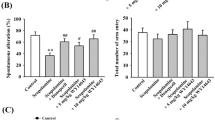
This study investigated the effects of alcoholic extract of Bacopa monnieri (L.) Wettst. (BM) on cognitive deficits using olfactory bulbectomized (OBX) mice and the underlying molecular mechanisms of its action. OBX mice were treated daily with BM (50 mg/kg, p.o.) or a reference drug, tacrine (2.5 mg/kg, i.p.), 1 week before and continuously 3 days after OBX. Cognitive performance of the animals was analyzed by the novel object recognition test, modified Y maze test, and fear conditioning test. Brain tissues of OBX animals were used for neurochemical and immunohistochemical studies. OBX impaired non-spatial short-term memory, spatial working memory, and long-term fair memory. BM administration ameliorated these memory disturbances. The effect of BM on short-term memory deficits was abolished by a muscarinic receptor antagonist, scopolamine. OBX downregulated phosphorylation of synaptic plasticity-related signaling proteins: NR1 subunit of N-methyl-d-aspartate receptor, glutamate receptor 1 (GluR1), and calmodulin-dependent kinase II but not cyclic AMP-responsive element binding protein (CREB), and reduced brain-derived neurotrophic factor (BDNF) mRNA in the hippocampus. OBX also reduced choline acetyltransferase in the hippocampus and cholinergic neurons in the medial septum, and enlarged the size of lateral ventricle. BM administration reversed these OBX-induced neurochemical and histological alterations, except the decrease of GluR1 phosphorylation, and enhanced CREB phosphorylation. Moreover, BM treatment inhibited ex vivo activity of acetylcholinesterase in the brain. These results indicate that BM treatment ameliorates OBX-induced cognition dysfunction via a mechanism involving enhancement of synaptic plasticity-related signaling and BDNF transcription and protection of cholinergic systems from OBX-induced neuronal damage.










Similar content being viewed by others
References
Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M (2010) Alzheimer’s disease: clinical trials and drug development. Lancet Neurol 9(7):702–716
Vollala VR, Upadhya S, Nayak S (2011) Learning and memory-enhancing effect of Bacopa monniera in neonatal rats. Bratisl Lek Listy 112(12):663–669
Vollala VR, Upadhya S, Nayak S (2011) Enhancement of basolateral amygdaloid neuronal dendritic arborization following Bacopa monniera extract treatment in adult rats. Clinics (Sao Paulo, Brazil) 66(4):663–671
Chatterjee M, Verma P, Palit G (2010) Comparative evaluation of Bacopa monniera and Panax quniquefolium in experimental anxiety and depressive models in mice. Indian J Exp Biol 48(3):306–313
Paulose CS, Chathu F, Khan SR, Krishnakumar A (2008) Neuroprotective role of Bacopa monnieri extract in epilepsy and effect of glucose supplementation during hypoxia: glutamate receptor gene expression. Neurochem Res 33(9):1663–1671
Mathew J, Gangadharan G, Kuruvilla KP, Paulose CS (2011) Behavioral deficit and decreased GABA receptor functional regulation in the hippocampus of epileptic rats: effect of Bacopa monnieri. Neurochem Res 36(1):7–16
Krishnakumar A, Abraham PM, Paul J, Paulose CS (2009) Down-regulation of cerebellar 5-HT(2C) receptors in pilocarpine-induced epilepsy in rats: therapeutic role of Bacopa monnieri extract. J Neurol Sci 284(1–2):124–128
Saraf MK, Prabhakar S, Anand A (2009) Bacopa monniera alleviates N(omega)-nitro-l-arginine arginine-induced but not MK-801-induced amnesia: a mouse Morris watermaze study. Neuroscience 160(1):149–155
Saraf MK, Prabhakar S, Anand A (2010) Neuroprotective effect of Bacopa monniera on ischemia induced brain injury. Pharmacol Biochem Behav 97(2):192–197
Saini N, Singh D, Sandhir R (2012) Neuroprotective effects of Bacopa monnieri in experimental model of dementia. Neurochem Res 37(9):1928–1937
Sathyanarayanan V, Thomas T, Einother SJ, Dobriyal R, Joshi MK, Krishnamachari S (2013) Brahmi for the better? New findings challenging cognition and anti-anxiety effects of Brahmi (Bacopa monniera) in healthy adults. Psychopharmacology 227:299–306
Morgan A, Stevens J (2010) Does Bacopa monnieri improve memory performance in older persons? Results of a randomized, placebo-controlled, double-blind trial. J Altern Complement Med 16(7):753–759
Peth-Nui T, Wattanathorn J, Muchimapura S, Tong-Un T, Piyavhatkul N, Rangseekajee P, Ingkaninan K, Vittaya-Areekul S (2012) Effects of 12-week Bacopa monnieri consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers. Evid Based Complement Alternat Med 2012:606424. doi:10.1155/2012/606424
Oba A, Nakagawasai O, Onogi H, Nemoto W, Yaoita F, Arai Y, Tan-No K, Tadano T (2013) Chronic fluvoxamine treatment changes 5-HT(2A/2C) receptor-mediated behavior in olfactory bulbectomized mice. Life Sci 92(2):119–124
Morales-Medina JC, Dumont Y, Bonaventure P, Quirion R (2012) Chronic administration of the Y2 receptor antagonist, JNJ-31020028, induced anti-depressant like-behaviors in olfactory bulbectomized rat. Neuropeptides 46(6):329–334
Yamada M, Hayashida M, Zhao Q, Shibahara N, Tanaka K, Miyata T, Matsumoto K (2011) Ameliorative effects of yokukansan on learning and memory deficits in olfactory bulbectomized mice. J Ethnopharmacol 135(3):737–746. doi:10.1016/j.jep.2011.04.010
Sithisarn P, Rojsanga P, Jarikasem S, Tanaka K, Matsumoto K (2013) Ameliorative effects of Acanthopanax trifoliatus on cognitive and emotional deficits in olfactory Bulbectomized mice: an animal model of depression and cognitive deficits. Evid Based Complement Alternat Med 2013:701956. doi:10.1155/2013/701956
Bahar-Fuchs A, Chetelat G, Villemagne VL, Moss S, Pike K, Masters CL, Rowe C, Savage G (2010) Olfactory deficits and amyloid-β burden in Alzheimer’s disease, mild cognitive impairment, and healthy aging: a PiB PET study. J Alzheimers Dis 22(4):1081–1087. doi:10.3233/JAD-2010-100696
Bahar-Fuchs A, Moss S, Rowe C, Savage G (2010) Olfactory performance in AD, aMCI, and healthy ageing: a unirhinal approach. Chem Senses 35(9):855–862. doi:10.1093/chemse/bjq094
Wesson DW, Levy E, Nixon RA, Wilson DA (2010) Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J Neurosci 30(2):505–514. doi:10.1523/JNEUROSCI.4622-09.2010
Aleksandrova IY, Kuvichkin VV, Kashparov IA, Medvinskaya NI, Nesterova IV, Lunin SM, Samokhin AN, Bobkova NV (2004) Increased level of beta-amyloid in the brain of bulbectomized mice. Biochemistry (Mosc) 69(2):176–180
Hozumi S, Nakagawasai O, Tan-No K, Niijima F, Yamadera F, Murata A, Arai Y, Yasuhara H, Tadano T (2003) Characteristics of changes in cholinergic function and impairment of learning and memory-related behavior induced by olfactory bulbectomy. Behav Brain Res 138(1):9–15
Horai H, Arita M, Kanaya S, Nihei Y, Ikeda T, Suwa K, Ojima Y, Tanaka K, Tanaka S, Aoshima K, Oda Y, Kakazu Y, Kusano M, Tohge T, Matsuda F, Sawada Y, Hirai MY, Nakanishi H, Ikeda K, Akimoto N, Maoka T, Takahashi H, Ara T, Sakurai N, Suzuki H, Shibata D, Neumann S, Iida T, Funatsu K, Matsuura F, Soga T, Taguchi R, Saito K, Nishioka T (2010) MassBank: a public repository for sharing mass spectral data for life sciences. J Mass Spectrom 45(7):703–714. doi:10.1002/jms.1777
Zhao Q, Murakami Y, Tohda M, Obi R, Shimada Y, Matsumoto K (2007) Chotosan, a Kampo formula, ameliorates chronic cerebral hypoperfusion-induced deficits in object recognition behaviors and central cholinergic systems in mice. J Pharmacol Sci 103:360–373
Ouchi H, Ono K, Murakami Y, Matsumoto K (2013) Social isolation induces deficit of latent learning performance in mice: a putative animal model of attention deficit/hyperactivity disorder. Behav Brain Res 238:146–153. doi:10.1016/j.bbr.2012.10.029
Inada C, Le Thi X, Tsuneyama K, Fujiwara H, Miyata T, Matsumoto K (2013) Endogenous acetylcholine rescues NMDA-induced long-lasting hippocampal cell damage via stimulation of muscarinic M(1) receptors: elucidation using organic hippocampal slice cultures. Eur J Pharmacol 699(1–3):150–159
Zhao Q, Matsumoto K, Tsuneyama K, Tanaka K, Li F, Shibahara N, Miyata T, Yokozawa T (2011) Diabetes-induced central cholinergic neuronal loss and cognitive deficit are attenuated by tacrine and a Chinese herbal prescription, kangen-karyu: elucidation in type 2 diabetes db/db mice. J Pharmacol Sci 117(4):230–242
Zhao Q, Yokozawa T, Tsuneyama K, Tanaka K, Miyata T, Shibahara N, Matsumoto K (2011) Chotosan (Diaoteng San)-induced improvement of cognitive deficits in senescence-accelerated mouse (SAMP8) involves the amelioration of angiogenic/neurotrophic factors and neuroplasticity systems in the brain. Chin Med 6:33. doi:10.1186/1749-8546-6-33
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ingkaninan K, Temkitthawon P, Chuenchom K, Yuyaem T, Thongnoi W (2003) Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J Ethnopharmacol 89(2–3):261–264
Bertaina-Anglade V, Enjuanes E, Morillon D, Drieu la Rochelle C (2006) The object recognition task in rats and mice: a simple and rapid model in safety pharmacology to detect amnesic properties of a new chemical entity. J Pharmacol Toxicol Methods 54(2):99–105
Saunders NL, Summers MJ (2009) Attention and working memory deficits in mild cognitive impairment. J Clin Exp Neuropsychol 32(4):350–357. doi:10.1080/13803390903042379
De Rosa R, Garcia AA, Braschi C, Capsoni S, Maffei L, Berardi N, Cattaneo A (2005) Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci USA 102(10):3811–3816. doi:10.1073/pnas.0500195102
Dellu F, Contarino A, Simon H, Koob GF, Gold LH (2000) Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiol Learn Mem 73(1):31–48
Csernansky JG, Martin M, Shah R, Bertchume A, Colvin J, Dong H (2005) Cholinesterase inhibitors ameliorate behavioral deficits induced by MK-801 in mice. Neuropsychopharmacology 30(12):2135–2143. doi:10.1038/sj.npp.1300761
Phillips RG, LeDoux JE (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106(2):274–285
Cooke SF, Bliss TV (2006) Plasticity in the human central nervous system. Brain 129(Pt 7):1659–1673
Primeaux SD, Holmes PV (1999) Role of aversively motivated behavior in the olfactory bulbectomy syndrome. Physiol Behav 67(1):41–47
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361(6407):31–39
Neves G, Cooke SF, Bliss TV (2008) Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci 9(1):65–75
Lau GC, Saha S, Faris R, Russek SJ (2004) Up-regulation of NMDAR1 subunit gene expression in cortical neurons via a PKA-dependent pathway. J Neurochem 88(3):564–575
Zhao H, Li Q, Pei X, Zhang Z, Yang R, Wang J, Li Y (2009) Long-term ginsenoside administration prevents memory impairment in aged C57BL/6 J mice by up-regulating the synaptic plasticity-related proteins in hippocampus. Behav Brain Res 201(2):311–317. doi:10.1016/j.bbr.2009.03.002
Chen BS, Roche KW (2007) Regulation of NMDA receptors by phosphorylation. Neuropharmacology 53(3):362–368
Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL (1996) Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16(6):1179–1188
Shaywitz AJ, Greenberg ME (1999) CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68:821–861. doi:10.1146/annurev.biochem.68.1.821
Lamprecht R (1999) CREB: a message to remember. Cell Mol Life Sci 55(4):554–563
Vaynman S, Ying Z, Gomez-Pinilla F (2007) The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience 144(3):825–833
Gomez-Pinilla F, Vaynman S, Ying Z (2008) Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci 28(11):2278–2287
Moriguchi S, Han F, Nakagawasai O, Tadano T, Fukunaga K (2006) Decreased calcium/calmodulin-dependent protein kinase II and protein kinase C activities mediate impairment of hippocampal long-term potentiation in the olfactory bulbectomized mice. J Neurochem 97(1):22–29
Han F, Nakano T, Yamamoto Y, Shioda N, Lu YM, Fukunaga K (2009) Improvement of depressive behaviors by nefiracetam is associated with activation of CaM kinases in olfactory bulbectomized mice. Brain Res 1265:205–214
Moriguchi S, Tanaka T, Tagashira H, Narahashi T, Fukunaga K (2013) Novel nootropic drug sunifiram improves cognitive deficits via CaM kinase II and protein kinase C activation in olfactory bulbectomized mice. Behav Brain Res 242:150–157
Lee I, Kesner RP (2003) Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J Neurosci 23(4):1517–1523
Yoon T, Okada J, Jung MW, Kim JJ (2008) Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learn Mem 15(3):97–105. doi:10.1101/lm.850808
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110
Zhao Q, Niu Y, Matsumoto K, Tsuneyama K, Tanaka K, Miyata T, Yokozawa T (2012) Chotosan ameliorates cognitive and emotional deficits in an animal model of type 2 diabetes: possible involvement of cholinergic and VEGF/PDGF mechanisms in the brain. BMC Complement Altern Med 12:188. doi:10.1186/1472-6882-12-188
Boyd TE, Trepel C, Racine RJ (2000) Cholinergic modulation of neocortical long-term potentiation in the awake, freely moving rat. Brain Res 881(1):28–36
Auerbach JM, Segal M (1996) Muscarinic receptors mediating depression and long-term potentiation in rat hippocampus. J Physiol 492(Pt 2):479–493
Blitzer RD, Gil O, Landau EM (1990) Cholinergic stimulation enhances long-term potentiation in the CA1 region of rat hippocampus. Neurosci Lett 119(2):207–210
Drever BD, Riedel G, Platt B (2011) The cholinergic system and hippocampal plasticity. Behav Brain Res 221(2):505–514
Acknowledgments
This work was in part supported by a Grant-in-Aid for the 2010 and 2012 Cooperative Research Project II from the Institute of Natural Medicine, University of Toyama (to H. T. N. P. and K. M.). L. T. X. is the recipient of a scholarship from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Le, X.T., Pham, H.T.N., Do, P.T. et al. Bacopa monnieri Ameliorates Memory Deficits in Olfactory Bulbectomized Mice: Possible Involvement of Glutamatergic and Cholinergic Systems. Neurochem Res 38, 2201–2215 (2013). https://doi.org/10.1007/s11064-013-1129-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1129-6