Abstract
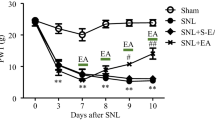
Previous studies indicated that disruption of glial function in the spinal cord enhanced electroacupuncture (EA) analgesia in arthritic rats, suggesting glia is involved in processing EA analgesia. To probe into the potential value for clinical practice, the present study was to investigate the effect of propentofylline, a glia inhibitor, on EA analgesia in rats. Mechanical allodynia induced by tetanic stimulation of sciatic nerve (TSS) was used as a pain model. On day 7 after TSS, EA treatment induced a significant increase in paw withdrawal threshold to mechanical stimulation. Intrathecal or intraperitoneal injection of propentofylline relieved TSS-induced mechanical allodynia. The combination of low dosage of propentofylline and EA produced more potent anti-allodynia than propentofylline or EA alone. Immunohistochemistry exhibited that TSS-induced activation of microglia and astrocytes was inhibited significantly by propentofylline. These results indicate that propentofylline and EA induce synergetic analgesia by interrupting spinal glial function.



Similar content being viewed by others
References
Guasti L, Richardson D, Jhaveri M, Eldeeb K, Barrett D, Elphick MR, Alexander SPH, Kendall D, Michael GJ, Chapman V (2009) Minocycline treatment inhibits microglial activation and alters spinal levels of endocannabinoids in a rat model of neuropathic pain. Mol Pain 5:35
Ziegler D (2008) Treatment of diabetic neuropathy and neuropathic pain how far have we come? Diabetes Care 31:S255–S261
Ying B, Lu N, Zhang YQ, Zhao ZQ (2006) Involvement of spinal glia in tetanically sciatic stimulation-induced bilateral mechanical allodynia in rats. Biochem Biophys Res Commun 340:1264–1272
Zhao ZQ (2008) Neural mechanism underlying acupuncture analgesia. Prog Neurobiol 85:355–375
Hwang BG, Min BI, Kim JH, Na HS, Park DS (2002) Effects of electroacupuncture on the mechanical allodynia in the rat model of neuropathic pain. Neurosci Lett 320:49–52
Dai Y, Kondo E, Fukuoka T, Tokunaga A, Miki K, Noguchi K (2001) The effect of electroacupuncture on pain behaviors and noxious stimulus-evoked Fos expression in a rat model of neuropathic pain. J Pain 2:151–159
Kim HN, Park JH, Kim SK, Sun B, Koo S, Choi SM, Bae H, Min BI (2008) Electroacupuncture potentiates the antiallodynic effect of intrathecal neostigmine in a rat model of neuropathic pain. J Physiol Sci 58:357–360
Kim SK, Park JH, Bae SJ, Kim JH, Hwang BG, Min BI, Park DS, Na HS (2005) Effects of electroacupuncture on cold allodynia in a rat model of neuropathic pain: mediation by spinal adrenergic and serotonergic receptors. Exp Neurol 195:430–436
Watkins LR, Milligan ED, Maier SF (2001) Spinal cord glia: new players in pain. Pain 93:201–205
Cao H, Zhang YQ (2008) Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev 32:972–983
Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, Zhang YQ (2007) New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain 129:64–75
DeLeo JA, Yezierski RP (2001) The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 90:1–6
Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L (2005) Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain 118:125–136
Sweitzer SM, Schubert P, DeLeo JA (2001) Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther 297:1210–1217
Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR (2005) Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 115:71–83
Sun S, Cao H, Han M, Li TT, Zhao ZQ, Zhang YQ (2008) Evidence for suppression of electroacupuncture on spinal glial activation and behavioral hypersensitivity in a rat model of monoarthritis. Brain Res Bull 75:83–93
Sun S, Chen WL, Wang PF, Zhao ZQ, Zhang YQ (2006) Disruption of glial function enhances electroacupuncture analgesia in arthritic rats. Exp Neurol 198:294–302
Bachynsky J, McCracken P, Lier D, Alloul K, Jacobs P (2000) Propentofylline treatment for Alzheimer disease and vascular dementia: an economic evaluation based on functional abilities. Alzheimer Dis Assoc Disord 14:102–111
Kittner B (1999) Clinical trials of propentofylline in vascular dementia. European/Canadian propentofylline study group. Alzheimer Dis Assoc Disord 13(Suppl 3):S166–S171
Zhang XC, Zhang YQ, Zhao ZQ (2005) Involvement of nitric oxide in long-term potentiation of spinal nociceptive responses in rats. Neuroreport 16:1197–1201
Liang LL, Wang ZY, Ning L, Yang JL, Zhang YQ, Zhao ZQ (2010) Involvement of nerve injury and activation of peripheral glial cells in tetanic sciatic stimulation-induced persistent pain in rats. J Neurosci Res. doi:10.1002/jnr.22439
Wang ZY, Zhang YQ, Zhao ZQ (2006) Inhibition of tetanically sciatic stimulation-induced LTP of spinal neurons and Fos expression by disrupting glutamate transporter GLT-1. Neuropharmacology 51:764–772
Watkins LR, Milligan ED, Maier SF (2001) Glial activation: a driving force for pathological pain. Trends Neurosci 24:450–455
Xu ZF, Wu GC, Cao XD (2002) Effect of electroacupuncture on the expression of interlukin-1beta mRNA after transient focal cerebral ischemia. Acupunct Electrother Res 27:29–35
Ruzicka BB, Thompson RC, Watson SJ, Akil H (1996) Interleukin-1 beta-mediated regulation of mu-opioid receptor mRNA in primary astrocyte-enriched cultures. J Neurochem 66:425–428
Ruzicka BB, Akil H (1997) The interleukin-1beta-mediated regulation of proenkephalin and opioid receptor messenger RNA in primary astrocyte-enriched cultures. Neuroscience 79:517–524
Parkinson FE, Paterson AR, Young JD, Cass CE (1993) Inhibitory effects of propentofylline on [3H]adenosine influx. A study of three nucleoside transport systems. Biochem Pharmacol 46:891–896
Loram LC, Harrison JA, Sloane EM, Hutchinson MR, Sholar P, Taylor FR, Berkelhammer D, Coats BD, Poole S, Milligan ED, Maier SF, Rieger J, Watkins LR (2009) Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: a novel therapy for neuropathic pain. J Neurosci 29:14015–14025
Sweitzer SM, Schubert P, DeLeo JA (2001) Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther 297:1210–1217
Liu C, Zhao F, Li W, Zhu L (1994) Role of adenosine in weak electro-acupuncture-induced depression of nociceptive response of spinal dorsal horn neurons in rats. Zhen Ci Yan Jiu 19:52–55
Lavand’homme PM, Eisenach JC (1999) Exogenous and endogenous adenosine enhance the spinal antiallodynic effects of morphine in a rat model of neuropathic pain. Pain 80:31–36
Acknowledgments
This work was supported by grants from National Basic Research Program of China (No. 2007CB512502 and 2007CB512303) and grants from National Nature Science Found of China (No. 30600178 and 30830044).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ling-Li Liang, Jia-Le Yang contributed equally.
Rights and permissions
About this article
Cite this article
Liang, LL., Yang, JL., Lü, N. et al. Synergetic Analgesia of Propentofylline and Electroacupuncture by Interrupting Spinal Glial Function in Rats. Neurochem Res 35, 1780–1786 (2010). https://doi.org/10.1007/s11064-010-0244-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0244-x