Abstract
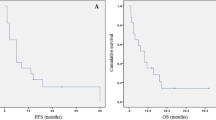
Fotemustine at the conventional dose of 100 mg/m2 is an active treatment for recurrent malignant gliomas (RMGs). However, it is associated with a relevant incidence of severe myelotoxicity, which is not justified in the palliative setting of this disease. This study was conducted to address whether administration of fotemustine at 60 mg/m2 (induction) followed by 75 mg/m2 (maintenance) would preserve clinical activity with the advantage of improved tolerance. Forty patients with RMGs pretreated with ≤2 lines of chemotherapy were enrolled. Median age was 57 years (26–80) and median Karnofsky performance status was 80 (60–100). Thirty-one patients (77.5%) had tissue available for analysis of the O6-methylguanine methyltransferase (MGMT) gene promoter which was found to be methylated in 14 cases (45%). Overall, 8 partial responses (20%) and 13 disease stabilizations (32.5%) were observed for a disease-control rate of 52.5%. At 6 months, 21% of patients were free from progression. Grades 3 and 4 platelet and white blood cell toxicity occurred in ≤10% of patients, and no patients discontinued treatment because of toxicity. No significant difference was observed for disease control rate between methylated and unmethylated patients, although a trend toward improved progression-free survival was reported for methylated patients. Low-dose fotemustine has activity comparable with that of the full-dose regimen, therefore it should be preferred for its greater tolerability. The role of MGMT gene promoter methylation status in relation to sensitivity to fotemustine is still unclear and needs further evaluation in future clinical trials.


Similar content being viewed by others
References
De Vita VT, Hellman S, Rosenberg SA (2008) Cancer, principles & practice of oncology, 8th edn. Lippincott Williams & Wilkins, Philadelphia
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Hayes MT, Bartley J, Parsons PG, Eaglesham GK, Prakash AS (1997) Mechanism of action of fotemustine, a new chloroethylnitrosourea anticancer agent: evidence for the formation of two DNA-reactive intermediates contributing to cytotoxicity. Biochemistry 36:10646–10654
Levin VA (1980) Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. Med Chem 23:682–684
Meulemans A, Giroux B, Hannoun P, Robine D, Henzel D (1991) Comparative diffusion study of two nitrosoureas: carmustine and fotemustine in normal rat brain, human and rat brain biopsies. Chemotherapy 37:86–92
Frenay M, Giroux B, Khoury S, Derlon JM, Namer M (1991) Phase II study of fotemustine in recurrent supratentorial malignant gliomas. Eur J Cancer 27:852–856
Malhaire JP, Lucas B, Simon H, Person H, Dam-Hieu P, Labat JP (1999) Fotemustine (Muphoran) in 22 patients with relapses of high-grade cerebral gliomas. Bull Cancer 86:289–294
Trevisan E, Laguzzi E, Ruda R, Guarneri D, Soffietti R (2008) Safety and efficacy of fotrmustine in recurrent or progressive gliomas. J Neurol 255:93 (abstract)
Khayat D, Lokiec F, Bizzari JP, Weil M, Meeus L, Sellami M, Rouesse J, Banzet P, Jacquillat C (1987) Phase I clinical study of the new amino acid-linked nitrosourea, S 10036, administered on a weekly schedule. Cancer Res 47:6782–6785
Brandes AA, Tosoni A, Franceschi E, Blatt V, Santoro A, Faedi M, Amistà P, Gardiman M, Labianca R, Bianchini C, Ermani M, Reni M (2009) Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: a phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Cancer Chemother Pharmacol 64:769–775
Fabi A, Metro G, Russillo M, Vidiri A, Carapella CM, Maschio M, Cognetti F, Jandolo B, Mirri MA, Sperduti I, Telera S, Carosi M, Pace A (2009) Treatment of recurrent malignant gliomas with fotemustine monotherapy: impact of dose and correlation with MGMT promoter methylation. BMC Cancer 9:101
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Available at: http://ctep.cancer.gov. Last Accessed 1 Nov 2009
A’Hern RP (2001) Sample size tables for exact single-stage phase II designs. Stat Med 20:859–866
Fabrini MG, Silvano G, Lolli I, Perrone F, Marsella A, Scotti V, Cionini L (2009) A multi-institutional phase II study on second-line Fotemustine chemotherapy in recurrent glioblastoma. J Neurooncol 92:79–86
Scoccianti S, Detti B, Sardaro A, Iannalfi A, Meattini I, Leonulli BG, Borghesi S, Martinelli F, Bordi L, Ammannati F, Biti G (2008) Second-line chemotherapy with fotemustine in temozolomide-pretreated patients with relapsing glioblastoma: a single institution experience. Anticancer Drugs 19:613–620
Taal W, Brandsma D, de Bruin HG, Bromberg JE, Swaak-Kragten AT, Smitt PA, van ES CA, van den Bent MJ (2008) Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113:405–410
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, Frezza G, Leonardi M, Spagnolli F, Ermani M (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192–2197
Fabi A, Russillo M, Metro G, Vidiri A, Di Giovanni S, Cognetti F (2009) Pseudoprogression and MGMT status in glioblastoma patients: implications in clinical practice. Anticancer Res 29:2607–2610
Fazeny-Dörner B, Veitl M, Wenzel C, Piribauer M, Rössler K, Dieckmann K, Ungersböck K, Marosi C (2003) Second-line chemotherapy with dacarbazine and fotemustine in nitrosourea-pretreated patients with recurrent glioblastoma multiforme. Anticancer Drugs 14:437–442
Silvani A, Lamperti E, Gaviani P, Eoli M, Fiumani A, Salmaggi A, Falcone C, Filippini G, Botturi A, Boiardi A (2008) Salvage chemotherapy with procarbazine and fotemustine combination in the treatment of temozolomide treated recurrent glioblastoma patients. J Neurooncol 87:143–151
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Soffietti R, Rudà R, Trevisan E, Picco E, Guarneri D, Caroli M, Fabrini M, Scotti V (2009) Phase II study of bevacizumab and nitrosourea in patients with recurrent malignant glioma: a multicenter Italian study. J Clin Oncol 27:15s (suppl; abstr 2012)
Acknowledgments
The authors gratefully acknowledge the advice of Dr. Antonio Silvani and Dr. Roberta Rudà.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fabi, A., Metro, G., Vidiri, A. et al. Low-dose fotemustine for recurrent malignant glioma: a multicenter phase II study. J Neurooncol 100, 209–215 (2010). https://doi.org/10.1007/s11060-010-0163-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0163-3