Abstract
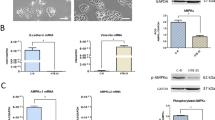
To understand the underlying pharmacological basis and the molecular mechanism of Taxol in therapy of cervical carcinoma (CC) disease, we need to explore the effect of Taxol on CC-related genes and pro-apoptosis and anti-apoptosis genes expression. Immunohistochemistry, western blot and reverse transcription-polymerase chain reaction were applied to examine postive expression levels of Bcl-2, Bax and Caspase-3, HGF, MACC1, Caspase-3 and C-met proteins and MACC1 mRNA expression in tumour of CC mice. Results showed that treatment of Taxol could increase the inhibition rate of tumour growth, positive expression levels of Caspase-3, Bax and decrease positive expression levels of Bcl-2 and Bcl-2/Bax, expression levels of HGF, MACC1 and C-met proteins and MACC1 mRNA in tumour tissue of CC mice. It can be concluded that inhibitory activity of Taxol against tumour growth in CC mice is closely associated with its modulating positive expression of Bcl-2, Bax, Caspase-3, expression of HGF, MACC1, Caspase-3 and C-met proteins and MACC1 mRNA in tumour of CC mice. In conclusion, HGF, MACC1 and C-met genes involve into malignant cervical tumors occurrence, development and prognosis, and might become potential molecular target therapy site of cervical cancer. Taxol intervention may serve as a multi-targeted CC therapeutic capable of inducing selective cancer cell death.








Similar content being viewed by others
References
Noordhuis MG, Eijsink JJ, Roossink F, de Graeff P, Pras E, Schuuring E, Wisman GB, de Bock GH, van der Zee AG (2011) Prognostic cell biological markers in cervical cancer patients primarily treated with (chemo) radiation: a systematic review. Int J Radiat Oncol 79:325–334
Ellenson LH, Wu TC (2004) Focus on endometrial and cervical cancer. Cancer Cell 5:533–538
Eifel PJ, Burke TW, Morris M, Smith TL (1995) Adenocarcinoma as an independent risk factor for disease recurrence in patients with stage IB cervical carcinoma. Gynecol Oncol 59:38–44
Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Parkin DM (1998) International trends in the incidence of cervical cancer: i. adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer 75:536–545
Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Borras J, Parkin DM (2000) International trends in incidence of cervical cancer: II. squamous-cell carcinoma. Int J Cancer 86:429–435
Vinh-Hung V, Bourgain C, Vlastos G, Cserni G, De Ridder M, Storme G, Vlastos AT (2007) Prognostic value of histopathology and trends in cervical cancer: a SEER population study. BMC Cancer 7:164
Horwitz SB (1992) Mechanism of action of taxol. Trends Pharmacol Sci 13:131–136
Rao S, Krauss NE, Heerding JM, Swindell CS, Ringell L, Orr GA, Horwitz SB (1994) 30-(p-Azidobenzamido)taxol photolabels the N-terminal 31 amino acids of beta-tubulin. J Biol Chem 269:3131–3134
Rao S, Orr GA, Chaudhary AG, Kingston DGY, Horwitz SB (1995) Characterization of the taxol binding site on the microtubule. 2-(m-Azidobenzoyl) taxol photolabels a peptide (amino acids 217–231) of beta-tubulin. J Biol Chem 270:20235–20238
Jordan MA, Wilson L (1998) Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol 10:123–130
Caplow M, Shanks J, Ruhlen R (1994) How taxol modulates microtubule disassembly. J Biol Chem 38:23399–23402
Horwitz SB (1994) Taxol (paclitaxel): mechanisms of action. Ann Oncol 6:S3–S6
Woods CM, Zhu J, McQueney PA, Bollag D, Lazarides E (1995) Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol Med 5:506–526
Rowinsky EK, Donehower RC (1995) Paclitaxel (taxol). N Engl J Med 15:004–1014
Eastman A, Rigas JR (1999) Modulation of apoptosis signaling pathways and cell cycle regulation. Semin Oncol 26:41–52
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326
Manon S, Chaudhuri B, Guérin M (1997) Release of cytochrome c and decrease of cytochrome c oxidase in Bax-expressing yeast cells, and prevention of these effects by coexpression of Bcl-xL. FEBS Lett 415:29–32
Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM (1998) Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci USA 95:554–559
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Stein U, Burock S, Herrmann P, Wendler I, Niederstrasser M (2012) Circulating MACC1 transcripts in colorectal cancer patient plasma predict metastasis and prognosis. PLoS One 7:e49249
Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, Birchmeier W, Schlag PM (2009) MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med 15:59–67
Shimokawa H, Uramoto H, Onitsuka T, Chundong G, Hanagiri T, Oyama T, Yasumoto K (2011) Overexpression of MACC1 mRNA in lung adenocarcinoma is associated with postoperative recurrence. J Thorac Cardiovasc Surg 141:895–898
Chundong G, Uramoto H, Onitsuka T, Shimokawa H, Iwanami T, Nakagawa M, Oyama T, Tanaka F (2011) Molecular diagnosis of MACC1 status in lung adenocarcinoma by immunohistochemical analysis. Anticancer Res 31:1141–1145
Shirahata A, Fan W, Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y, Hibi K (2011) MACC 1 as a marker for vascular invasive hepatocellular carcinoma. Anticancer Res 31:777–780
Zhang R, Shi H, Chen Z, Wu Q, Ren F, Huang H (2011) Effects of metastasis-associated in colon cancer 1 inhibition by small hairpin RNA on ovarian carcinoma OVCAR-3 cells. J Exp Clin Cancer Res 30:83
Shirahata A, Sakata M, Kitamura Y, Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K (2010) MACC 1 as a marker for peritoneal-disseminated gastric carcinoma. Anticancer Res 30:3441–3444
Nakamura T, Nawa K, Ichihara A (1984) Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun 122:1450–1459
Russell WE, McGowan JA, Bucher NL (1984) Partial characterization of a hepatocyte growth factor from rat platelets. J Cell Physiol 119:183–192
Luetteke NC, Michalopoulos GK (1985) Partial purification and characterization of a hepatocyte growth factor produced by rat hepatocellular carcinoma cells. Cancer Res 45:6331–6337
Nakamura T, Teramoto H, Ichihara A (1986) Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci USA 83:6489–6493
Nakamura T, Nawa K, Ichihara A, Kaise N, Nishino T (1987) Purification and subunit structure of hepatocyte growth factor from rat platelets. FEBS Lett 224:311–316
Gohda E, Tsubouchi H, Nakayama H, Hirono S, Sakiyama O, Takahashi K, Miyazaki H, Hashimoto S, Daikuhara Y (1988) Purification and partial characterization of hepatocyte growth factor from plasma of a patient with fulminant hepatic failure. J Clin Invest 81:414–419
Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, Vande Woude GF (1984) Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 311:29–33
Navab R, Liu J, Seiden-Long I, Shih W, Li M, Bandarchi B, Chen Y, Lau D, Zu YF, Cescon D, Zhu CQ, Organ S, Ibrahimov E, Ohanessian D, Tsao MS (2009) Co-overexpression of Met and hepatocyte growth factor promotes systemic metastasis in NCI-H460 non-small cell lung carcinoma cells. Neoplasia 11:1292–1300
Yi S, Tsao MS (2000) Activation of hepatocyte growth factor-met autocrine loop enhances tumorigenicity in a human lung adenocarcinoma cell line. Neoplasia 2:226–234
Yi S, Chen JR, Viallet J, Schwall RH, Nakamura T, Tsao MS (1998) Paracrine effects of hepatocyte growth factor/scatter factor on non-small-cell lung carcinoma cell lines. Br J Cancer 77:2162–2170
Rong S, Segal S, Anver M, Resau JH, Vande Woude GF (1994) Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci USA 91:4731–4735
Tsao MS, Zhu H, Giaid A, Viallet J, Nakamura T, Park M (1993) Hepatocyte growth factor/scatter factor is an autocrine factor for human normal bronchial epithelial and lung carcinoma cells. Cell Growth Differ 4:571–579
Date K, Matsumoto K, Kuba K, Shimura H, Tanaka M, Nakamura T (1998) Inhibition of tumor growth and invasion by a four-kringle antagonist (HGF/NK4) for hepatocyte growth factor. Oncogene 17:3045–3054
Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T (1997) HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett 420:1–6
Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, Birchmeier W, Schlag PM (2009) MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med 15:59–67
Stein U, Arlt F, Walther W, Smith J, Waldman T, Harris ED, Mertins SD, Heizmann CW, Allard D, Birchmeier W, Schlag PM, Shoemaker RH (2006) The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology 131:1486–1500
Galimi F, Torti D, Sassi F, Isella C, Corà D (2011) Genetic and expression analysis of MET, MACC1, and HGF in metastatic colorectal cancer: response to met inhibition in patient xenografts and pathologic correlations. Clin Cancer Res 17:3146–3156
Stein U, Smith J, Walther W, Arlt F (2009) MACC1 controls Met: what a difference an Sp1 site makes. Cell Cycle 8:2467–2469
Gherardi E, Birchmeier W, Birchmeier C, Woude GV (2012) Targeting MET in cancer: rationale and progress. Nat Rev Cancer 12:89–103
Kitamura YH, Shirahata A, Sakata M, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y, Hibi K (2009) Frequent methylation of vimentin in well-differentiated gastric carcinoma. Anticancer Res 29(6):2227–2229
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiao-Ping Chen and Xin-Ping Ren have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Chen, XP., Ren, XP., Lan, JY. et al. Analysis of HGF, MACC1, C-met and apoptosis-related genes in cervical carcinoma mice. Mol Biol Rep 41, 1247–1256 (2014). https://doi.org/10.1007/s11033-013-2969-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2969-5