Abstract
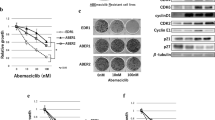
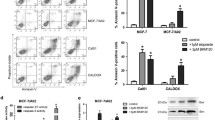
Despite the improvement of strategies against cancer therapy, the multidrug resistance (MDR)is the critical problem for successful cancer therapy. Recurrent cancers after initial treatment with chemotherapy are generally refractory to second treatments with these anticancer therapies. Therefore, it is necessary to elucidate the therapy-resistant mechanism for development of effective therapeutic modalities against tumors. Here we demonstrate a phase-specific chemotherapy resistance due to epidermal growth factor receptor (EGFR) in human breast cancer cells. Thymidine-induced G1-arrested cultures showed upregulated chemosensitivity, whereas S-phase arrested cells were more resistant to chemotherapeutic agents. Overexpression of EGFR promoted the MDR phenotypes in breast cancer cells via accelerating the G1/S phase transition, whereas depletion of EGFR exerted the opposite effects. Furthermore, CyclinD1, a protein related to cell cycle, was demonstrated to be involved in above EGFR-mediated effects since EGFR increased the expression of CyclinD1, and the specific RNA interference against CyclinD1 could primarily abolish the EGFR-induced MDR phenotypes. These data provide new insights into the mode by which MDR breast cancers evade cytoxic attacks from chemotherapeutic agents and also suggest a role for EGFR-CyclinD1 axis in this process.





Similar content being viewed by others
References
Stavrovskaya AA (2000) Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry 65:95–106
Eytan GD (2005) Mechanism of multidrug resistance in relation to passive membrane permeation. Biomed Pharmacother 59:90–97. doi:10.1016/j.biopha.2005.01.003
Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F (2006) The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR). Curr Drug Targets 7:893–909. doi:10.2174/138945006777709520
Chekhun VF, Lukyanova NY, Yurchenko OV, Kulik GI (2005) The role of expression of the components of proteome in the formation of molecular profile of human ovarian carcinoma A2780 cells sensitive and resistant to cisplatin. Exp Oncol 27:191–195
Chekhun VF, Ganina KP, Kulik GI, Solianik GI, Kunskaia LN, Gushchenko NN (2000) Influence of tumor drug resistance phenotype on the dynamics of cisplatin-induced changes of rat peripheral lymphocyte chromatin structure in Guerin’s carcinoma. Tsitol Genet 34:11–17
Motwani M, Delohery TM, Schwartz GK (1999) Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin Cancer Res 5:1876–1883
Motwani M, Schwartz GK (1998) Inappropriate cell cycle progression enhances the induction of apoptosis by flavopiridol in Taxol treated gastric cancer cells. Proc Am Assoc Cancer Res 39:190
Zhou Y, Ling XL, Li SW, Li XQ, Yan B (2010) Establishment of a human hepatoma multidrug resistant cell line in vitro. World J Gastroenterol 16:2291–2297. doi:10.3748/wjg.v16.i18.2291
Zhuang DX, Liu YC, Ying Mao et al (2011) TMZ-induced PrPc/par-4 interaction promotes the survival of human glioma cells. Int J Cancer. doi:10.1002/ijc.25985
Mirski SE, Gerlach JH, Cole SP (1987) Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res 47:2594–2598
Li QQ, Wang WJ, Xu JD et al (2007) Up-regulation of CD147 and matrix metalloproteinase-2, -9 induced by P-glycoprotein substrates in multidrug resistant breast cancer cells. Cancer Sci 98:1767–1774. doi:10.1111/j.1349-7006.2007.00593.x
Li QQ, Chen ZQ, Xu JD et al (2010) Overexpression and involvement of special AT-rich sequence binding protein 1 in multidrug resistance in human breast carcinoma cells. Cancer Sci 101:80–86. doi:10.1111/j.1349-7006.2009.01372.x
Srivastava SK, Singh S (2004) Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferation activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis 25:1701–1709. doi:10.1093/carcin/bgh179
Meyers MB, Merluzzi VJ, Spengler BA, Biedler JL (1986) Epidermal growth factor receptor is increased in multidrug-resistant Chinese hamster and mouse tumor cells. Proc Natl Acad Sci 83:5521–5525. doi:10.1073/pnas.83.15.5521
Meyers MB, Shen WP, Spengler BA et al (1988) Epidermal growth factor receptor in multidrug-resistant human neuroblastoma cells. J Cell Biochem 38:87–97. doi:10.1002/jcb.240380203
Sherr CJ (1994) G1 phase progression: cycling on cue. Cell 79:551–555. doi:10.1016/0092-8674(94)90540-1
Kaufmann WK, Paules RS (1996) DNA damage and cell cycle checkpoints. FASEB J 10:238–247
Herbst RS (2004) Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys 59:21–26. doi:10.1016/j.ijrobp.2003.11.041
Garcia R, Franklin RA, McCubrey JA (2006) EGF induces cell motility and multi-drug resistance gene expression in breast cancer cells. Cell Cycle 5:2820–2826. doi:10.4161/cc.5.23.3535
Kitazaki T, Oka M, Nakamura Y et al (2005) Gefitinib, an EGFR tyrosine kinase inhibitor, directly inhibits the function of P-glycoprotein in multidrug resistant cancer cells. Lung Cancer 49:337–343. doi:10.1016/j.lungcan.2005.03.035
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (No. 81000957). We thank members of our laboratory for helpful discussions.
Conflict of interest
We have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Shu-Jun Chen and Jing Luan contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Chen, SJ., Luan, J., Zhang, HS. et al. EGFR-mediated G1/S transition contributes to the multidrug resistance in breast cancer cells. Mol Biol Rep 39, 5465–5471 (2012). https://doi.org/10.1007/s11033-011-1347-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1347-4