Abstract
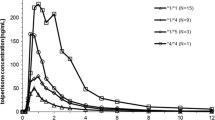
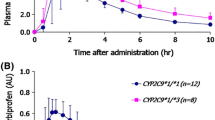
To relate the pharmacokinetics of orally administered lansoprazole in healthy adult Jordanian men with CYP2C19 polymorphisms and to determine the percentage of CYP2C19 polymorphism in Jordanian population and the allelic frequency of CYP2C19*2 and CYP2C19*3. A total of 78 healthy Jordanian volunteers were included in this study from three different bioequivalence studies, one of these studies which included 26 volunteers was done on lansoprazole. Genotyping for CYP2C19*1, CYP2C19*2, CYP2C19*3 was done for all 78 volunteers, the data of genotyping of all subjects used for screening the frequency of different genotypes and the allelic frequency of different polymorphisms in healthy Jordanian men, the pharmacokinetics and genotyping data for the study of lansoprazole was matched and compared to investigate presence of statistical differences in pharmacokinetic parameters. In Jordanian subjects, the allele frequencies of the CYP2C19*2 and CYP2C19*3 mutation were 0.16 and 0, respectively. The concentration–time curves in the two groups [homozygote extensive metabolizer (homEM, n = 19) and heterozygote extensive metabolizer (homEM, n = 7)] groups were fitted to a non-compartment model. In the homEM and in the hetEM groups, the main kinetic parameters were as follows: Tmax (2.1875 ± 0.777) and (2.54 ± 1.87) h, Cmax (697.875 ± 335) and (833.58 ± 436.26) mg/l, t1/2 (1.3 ± 0.43) and (2.38 ± 1.64) h, AUC(0→∞) were (1,684.9 ± 888) and (3,609.8 ± 318) mg h l−1, respectively. The Jordanian population showed similarities in CYP2C19 allele and genotype distribution pattern with Caucasians and Africans. CYP2C19 allele and poor metabolizer (PM) genotype frequencies in the Jordanian population are distinct from populations’ from East Asia such as Japanese and Koreans. Although lower pharmacokinetic parameters were found in homEM compared to hetEM but there was no significant difference between the two groups (P < 0.05).

Similar content being viewed by others
References
Li-Wan-Po A, Girard T, Farndon P, Cooley C, Lithgow J (2010) Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19*17. Br J Clin Pharmacol 69(3):222–230
Myrand SP, Sekiguchi K, Man MZ, Lin X, Tzeng RY, Teng CH, Hee B, Garrett M, Kikkawa H, Lin CY, Eddy SM, Dostalik J, Mount J, Azuma J, Fujio Y, Jang IJ, Shin SG, Bleavins MR, Williams JA, Paulauskis JD, Wilner KD (2008) Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther 84(3):347–361
De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA (1994) Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol 46(4):594–598
Sachs G, Shin JM, Briving C, Wallmark B, Hersey S (1995) The pharmacology of the gastric acid pump: the H+, K+ ATPase. Annu Rev Pharmacol Toxicol 35:277–305
Sachs G (1997) Proton pump inhibitors and acid-related diseases. Pharmacotherapy 17(1):22–37
Kita T, Sakaeda T, Baba T, Aoyama N, Kakumoto M, Kurimoto Y, Kawahara Y, Okamura N, Kirita S, Kasuga M, Okumura K (2003) Different contribution of CYP2C19 in the in vitro metabolism of three proton pump inhibitors. Biol Pharm Bull 26(3):386–390
Chong E, Ensom MH (2003) Pharmacogenetics of the proton pump inhibitors: a systematic review. Pharmacotherapy 23(4):460–471
Andersson T, Regardh CG, Lou YC, Zhang Y, Dahl ML, Bertilsson L (1992) Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics 2(1):25–31
Jacqz E, Dulac H, Mathieu H (1988) Phenotyping polymorphic drug metabolism in the French Caucasian population. Eur J Clin Pharmacol 35(2):167–171
Kubota T, Chiba K, Ishizaki T (1996) Genotyping of S-mephenytoin 4′-hydroxylation in an extended Japanese population. Clin Pharmacol Ther 60(6):661–666
Shu Y, Zhou HH (2000) Individual and ethnic differences in CYP2C19 activity in Chinese populations. Acta Pharmacol Sin 21(3):193–199
de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA (1994) The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 269(22):15419–15422
Adachi K, Katsube T, Kawamura A, Takashima T, Yuki M, Amano K, Ishihara S, Fukuda R, Watanabe M, Kinoshita Y (2000) CYP2C19 genotype status and intragastric pH during dosing with lansoprazole or rabeprazole. Aliment Pharmacol Ther 14(10):1259–1266
Sameer AE, Amany GM, Abdela AA, Fadel SA (2009) CYP2C19 genotypes in a population of healthy volunteers and in children with hematological malignancies in Gaza Strip. Can J Clin Pharmacol 16(1):e156–e162
Robarge J, Fletcher R, Nguyen A, Thorn C (2006) Important Haplotype Information for CYP2C19. Pharmacogenomics Knowledge Base. http://www.pharmgkb.org/search/annotatedGene/cyp2c19/haplotype.jsp. Last modified 30 Sep 2006
Hamdy SI, Hiratsuka M, Narahara K, El-Enany M, Moursi N, Ahmed MS, Mizugaki M (2002) Allele and genotype frequencies of polymorphic cytochromes P450 (CYP2C9, CYP2C19, CYP2E1) and dihydropyrimidine dehydrogenase (DPYD) in the Egyptian population. Br J Clin Pharmacol 53(6):596–603
Goldstein JA, Ishizaki T, Chiba K, de Morais SM, Bell D, Krahn PM, Evans DA (1997) Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics 7(1):59–64
Djaffar Jaffar Jureidini I, Chamseddine N, Keleshian S, Naoufal R, Zahed L, Hakime N (2011) Prevalence of CYP2C19 polymorphisms in the Lebanese population. Mol Biol Rep [Epub ahead of print]
Dugger HA, Carlson JD, Henderson W, Erdmann GR, Alam SM, Dham R, Quamruzaman (2001) Bioequivalence evaluation of lansoprazole 30-mg capsules (Lanfast and Lanzor) in healthy volunteers. Eur J Pharm Biopharm 51(2):153–157
Karol MD, Granneman GR, Alexander K (1995) Determination of lansoprazole and five metabolites in plasma by high-performance liquid chromatography. J Chromatogr B 668(1):182–186
Oliveira CH, Barrientos-Astigarraga RE, Abib E, Mendes GD, da Silva DR, de Nucci G (2003) Lansoprazole quantification in human plasma by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B 783(2):453–459
Ghodke Y, Joshi K, Arya Y, Radkar A, Chiplunkar A, Shintre P, Patwardhan B (2007) Genetic polymorphism of CYP2C19 in Maharashtrian population. Eur J Epidemiol 22(12):907–915
Furuta T, Shirai N, Watanabe F, Honda S, Takeuchi K, Iida T, Sato Y, Kajimura M, Futami H, Takayanagi S, Yamada M, Ohashi K, Ishizaki T, Hanai H (2002) Effect of cytochrome P4502C19 genotypic differences on cure rates for gastroesophageal reflux disease by lansoprazole. Clin Pharmacol Ther 72(4):453–460
Saitoh T, Otsuka H, Kawasaki T, Endo H, Iga D, Tomimatsu M, Fukushima Y, Katsube T, Ogawa K, Otsuka K (2009) Influences of CYP2C19 polymorphism on recurrence of reflux esophagitis during proton pump inhibitor maintenance therapy. Hepatogastroenterology 56(91–92):703–706
Ward MB, Foster DJ (2011) CYP2C19-guided design of a proton pump inhibitor dose regimen to avoid the need for pharmacogenetic individualization in H. pylori eradication. Eur J Clin Pharmacol 67(3):261–266
Acknowledgments
This work was done as part of the MSc thesis sponsored by the Deanship of Higher Education, The University of Jordan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zalloum, I., Hakooz, N. & Arafat, T. Genetic polymorphism of CYP2C19 in a Jordanian population: influence of allele frequencies of CYP2C19*1 and CYP2C19*2 on the pharmacokinetic profile of lansoprazole. Mol Biol Rep 39, 4195–4200 (2012). https://doi.org/10.1007/s11033-011-1204-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1204-5