Abstract
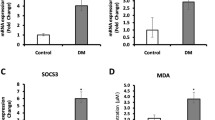
Although subclinical inflammation and oxidative stress are implicated in the aetiology of diabetes, there are hardly any studies in prediabetes. Therefore, we made an attempt to study the gene expression pattern of certain inflammatory/oxidative genes using lymphocytes from Type 2 diabetic patients, impaired glucose tolerance (IGT), and normal glucose tolerance (NGT) subjects. Compared to NGT group, interleukin-6, tumor necrosis factor-α (TNF-α), p22Phox NADPH oxidase, and thioredoxin interacting protein (TXNIP) mRNA levels were higher and suppressor of cytokine signaling (SOCS-3) mRNA was lower in subjects with IGT and diabetes. The mean (±SE) levels of thiobarbituric acid reactive substances and protein carbonyl content were also elevated in glucose intolerant subjects. In multiple linear regression analysis, TXNIP and TNF-α showed a significant association with HbA1c even after adjusting for TBARS and PCO (TXNIP: β = 1.70, P < 0.01; TNF-α: β = 1.86, P < 0.01). Increased subclinical inflammation/oxidation is seen in Asian Indians with not only Type 2 diabetes but also IGT.



Similar content being viewed by others
References
Gokulakrishnan K, Deepa R, Mohan V (2008) Association of high sensitivity C-reactive protein [hsCRP] and tumour necrosis factor-alpha [TNF-alpha] with carotid intimal medial thickness in subjects with different grades of glucose intolerance—the Chennai urban rural epidemiology study (CURES-31). Clin Biochem 41:480–485
Deepa R, Velmurugan K, Arvind K et al (2006) Serum levels of interleukin 6, C-reactive protein, vascular cell adhesion molecule 1, and monocyte chemotactic protein 1 in relation to insulin resistance and glucose intolerance—the Chennai urban rural epidemiology study (CURES). Metabolism 55:1232–1238. doi:10.1016/j.metabol.2006.05.008
Sampathkumar R, Balasubramanyam M, Sudarslal S, Rema M, Mohan V, Balaram P (2005) Increased glutathionylated hemoglobin (HbSSG) in type 2 diabetes subjects with microangiopathy. Clin Biochem 38:892–899. doi:10.1016/j.clinbiochem.2005.06.009
Adaikalakoteswari A, Balasubramanyam M, Mohan V (2005) Telomere shortening occurs in Asian Indian type 2 diabetic patients. Diabet Med 22:1151–1156. doi:10.1111/j.1464-5491.2005.01574.x
Knowler WC, Narayan KM, Hanson RL et al (1995) Preventing non-insulin-dependent diabetes. Diabetes 44:483–488. doi:10.2337/diabetes.44.5.483
Gokulakrishnan K, Deepa R, Velmurugan K, Ravikumar R, Karkuzhali K, Mohan V (2007) Oxidized low-density lipoprotein and intimal medial thickness in subjects with glucose intolerance: the Chennai urban rural epidemiology study-25. Metabolism 56:245–250. doi:10.1016/j.metabol.2006.10.002
Gokulakrishnan K, Deepa R, Mohan V, Gross MD (2006) Soluble P-selectin and CD40L levels in subjects with prediabetes, diabetes mellitus, and metabolic syndrome—the Chennai urban rural epidemiology study. Metabolism 55:237–242. doi:10.1016/j.metabol.2005.08.019
Donahue RP, Rejman K, Rafalson LB, Dmochowski J, Stranges S, Trevisan M (2007) Sex differences in endothelial function markers before conversion to pre-diabetes: does the clock start ticking earlier among women? the Western New York study. Diabetes Care 30:354–359. doi:10.2337/dc06-1772
Patti ME (2004) Gene expression in humans with diabetes and prediabetes: what have we learned about diabetes pathophysiology? Curr Opin Clin Nutr Metab Care 7:383–390. doi:10.1097/01.mco.0000134359.23288.72
Balasubramanyam M, Premanand C, Mohan V (2002) The lymphocyte as a cellular model to study insights into the pathophysiology of diabetes and its complications. Ann NY Acad Sci 958:399–402
Srinivasan V, Sandhya N, Sampathkumar R, Farooq S, Mohan V, Balasubramanyam M (2007) Glutamine fructose-6-phosphate amidotransferase (GFAT) gene expression and activity in patients with type 2 diabetes: inter-relationships with hyperglycaemia and oxidative stress. Clin Biochem 40:952–957. doi:10.1016/j.clinbiochem.2007.05.002
Stentz FB, Kitabchi AE (2007) Transcriptome and proteome expressions involved in insulin resistance in muscle and activated T-lymphocytes of patients with type 2 diabetes. Genomics Proteomics Bioinformatics 5:216–235. doi:10.1016/S1672-0229(08)60009-1
Robertson AK, Hansson GK (2006) T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol 26:2421–2432. doi:10.1161/01.ATV.0000245830.29764.84
Kintscher U, Hartge M, Hess K et al (2008) T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 28:1304–1310. doi:10.1161/ATVBAHA.108.165100
Deepa M, Pradeepa R, Rema M et al (2003) The Chennai urban rural epidemiology study (CURES)—study design and methodology (urban component) (CURES-I). J Assoc Physicians India 51:863–870
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus, provisional report of a WHO Consultation. Diabet Med 15:539–553. doi:10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Balasubramanyam M, Kimura M, Aviv A, Gardner JP (1993) Kinetics of calcium transport across the lymphocyte plasma membrane. Am J Physiol 265:C321–C327
Balasubramanyam M, Rohowsky-Kochan C, Reeves JP, Gardner JP (1994) Na+/Ca2+exchange-mediated calcium entry in human lymphocytes. J Clin Invest 94:2002–2008. doi:10.1172/JCI117553
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. doi:10.1016/0076-6879(90)86141-H
Yagi K (1976) A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med 15:212–216. doi:10.1016/0006-2944(76)90049-1
Gimeno RE, Klaman LD (2005) Adipose tissue as an active endocrine organ: recent advances. Curr Opin Pharmacol 5:122–128. doi:10.1016/j.coph.2005.01.006
Pickup JC, Mattock MB, Chusney GD, Burt D (1997) NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 40:1286–1292. doi:10.1007/s001250050822
Mohan V, Deepa R, Velmurugan K, Premalatha G (2005) Association of C-reactive protein with body fat, diabetes and coronary artery disease in Asian Indians: the Chennai urban rural epidemiology study (CURES-6). Diabet Med 22:863–870. doi:10.1111/j.1464-5491.2005.01541.x
Tsiotra PC, Tsigos C, Yfanti E et al (2007) Visfatin, TNF-alpha and IL-6 mRNA expression is increased in mononuclear cells from type 2 diabetic women. Horm Metab Res 39:758–763. doi:10.1055/s-2007-990288
Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S (2004) Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 24:2137–2142. doi:10.1161/01.ATV.0000143933.20616.1b
Sato K, Niessner A, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM (2006) TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J Exp Med 203:239–250. doi:10.1084/jem.20051062
Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95:2409–2415. doi:10.1172/JCI117936
Susa S, Daimon M, Sakabe J et al (2008) A functional polymorphism of the TNF-alpha gene that is associated with type 2 DM. Biochem Biophys Res Commun 369:943–947. doi:10.1016/j.bbrc.2008.02.121
Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW (1997) Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA 94:3195–3199. doi:10.1073/pnas.94.7.3195
Bouma G, Crusius JB, Oudkerk Pool M et al (1996) Secretion of tumour necrosis factor alpha and lymphotoxin alpha in relation to polymorphisms in the TNF genes and HLA-DR alleles. Relevance for inflammatory bowel disease. Scand J Immunol 43:456–463. doi:10.1046/j.1365-3083.1996.d01-65.x
Ruotsalainen E, Salmenniemi U, Vauhkonen I et al (2006) Changes in inflammatory cytokines are related to impaired glucose tolerance in offspring of type 2 diabetic subjects. Diabetes Care 29:2714–2720. doi:10.2337/dc06-0147
Rui L, Yuan M, Frantz D, Shoelson S, White MF (2002) SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 277:42394–42398. doi:10.1074/jbc.C200444200
Lebrun P, Van Obberghen E (2008) SOCS proteins causing trouble in insulin action. Acta Physiol (Oxf) 192:29–36
Yu CR, Mahdi RM, Ebong S, Vistica BP, Gery I, Egwuagu CE (2003) Suppressor of cytokine signaling 3 regulates proliferation and activation of T-helper cells. J Biol Chem 278:29752–29759. doi:10.1074/jbc.M300489200
Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR (2003) Pancreatic β-cells express phagocyte-like NAD(P). Hoxidase. Diabetes 52:1457–1463. doi:10.2337/diabetes.52.6.1457
Adaikalakoteswari A, Balasubramanyam M, Rema M, Mohan V (2006) Differential gene expression of NADPH oxidase (p22phox) and hemoxygenase-1 in patients with Type 2 diabetes and microangiopathy. Diabet Med 23:666–674. doi:10.1111/j.1464-5491.2006.01879.x
Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A (2008) Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes 57:938–944. doi:10.2337/db07-0715
Parikh H, Carlsson E, Chutkow WA et al (2007) TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 4:e158. doi:10.1371/journal.pmed.0040158
Junn E, Han SH, Im JY et al (2000) Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol 164:6287–6295
Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT (2004) Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem 279:30369–30374. doi:10.1074/jbc.M400549200
Yamawaki H, Pan S, Lee RT, Berk BC (2005) Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Invest 115:733–738
Wei XF, Zhou QG, Hou FF, Liu BY, Liang M (2008) Advanced oxidation protein products induce mesangial cells perturbation through PKC-dependent activation of NADPH oxidase. Am J Physiol Renal Physiol. doi:10.1152/ajprenal.90536.2008
Shi XY, Hou FF, Niu HX et al (2008) Advanced oxidation protein products promote inflammation in diabetic kidney through activation of renal nicotinamide adenine dinucleotide phosphate oxidase. Endocrinology 149:1829–1839. doi:10.1210/en.2007-1544
Hansson GK, Holm J, Jonasson L (1989) Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol 135:169–175
Hänninen A, Nurmela R, Maksimow M, Heino J, Jalkanen S, Kurts C (2007) Islet beta-cell-specific T cells can use different homing mechanisms to infiltrate and destroy pancreatic islets. Am J Pathol 170:240–250. doi:10.2353/ajpath.2007.060142
Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P (2008) Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res 103:467–476. doi:10.1161/CIRCRESAHA.108.177105
Acknowledgements
This work was supported by research grants from the Department of Science and Technology (DST-FIST), Indian Council of Medical Research (ICMR) and the Department of Biotechnology (DBT), Government of India, New Delhi, India. KG and FM acknowledges the financial assistance (Senior Research Fellowship) from the Council of Scientific & Industrial Research (CSIR), New Delhi, India. This is paper no. 49 from the Chennai Urban Rural Epidemiology Study (CURES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gokulakrishnan, K., Mohanavalli, K.T., Monickaraj, F. et al. Subclinical inflammation/oxidation as revealed by altered gene expression profiles in subjects with impaired glucose tolerance and Type 2 diabetes patients. Mol Cell Biochem 324, 173–181 (2009). https://doi.org/10.1007/s11010-008-9996-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9996-x