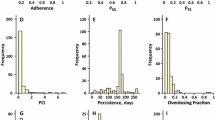
Aims: To present a method for analyzing side-effect data where change in severity is spontaneously reported during the experiment. Methods: A clinical study in 12 healthy volunteers aimed to investigate the concentration-response characteristics of a CNS-specific side-effect was conducted. After an open session where the subjects experienced the side-effect and where the individual pharmacokinetic parameters were evaluated they were randomized to a sequence of three different infusion rates of the drug in a double-blinded crossover way. The infusion rates were individualized to achieve the same target concentration in all subjects and different drug input rates were selected to mimic absorption profiles from different formulations. The occurrence of the specific side-effect and any subsequent change in severity was self-reported by the subjects. Severity was recorded as 0 = no side-effect, 1 = mild side-effect and 2 = moderate or severe side-effect. Results: The side-effect data were analyzed using a mixed-effects model for ordered categorical data with and without Markov elements. The former model estimated the probability of having a certain side-effect score conditioned on the preceding observation and drug exposure. The observed numbers of transitions between scores were from 0 −> 1: 24, from 0− > 2: 11, from 1 − >, 2: 23, from 2− > 1: 1, from 2− > 0: 32 and from 1 − >0: 2. The side-effect model consisted of an effect-compartment model with a tolerance compartment. The predictive performance of the Markov model was investigated by a posterior predictive check (PPC), where 100 datasets were simulated from the final model. Average number of the different transitions from the PPC was from 0 − > 1: 26, from 0 − > 2: 11, from 1 − > 2: 25, from 2 − >1: 1, from 2 − >0: 35 and from 1 − > 0: 1. A similar PPC for the model without Markov elements was at considerable disparity with the data. Conclusion: This approach of incorporating Markov elements in an analysis of spontaneously reported categorical side-effect data could adequately predict the observed side-effect time course and could be considered in analyses of categorical data where dependence between observations is an issue.
Similar content being viewed by others
References
L.B. Sheiner (1994) ArticleTitleA new approach to the analysis of analgesic drug trials, illustrated with bromfenac data Clin. Pharmacol. Ther. 56 IssueID3 309–322 Occurrence Handle7924127 Occurrence Handle1:STN:280:DyaK2M%2FgvVGquw%3D%3D Occurrence Handle10.1038/clpt.1994.142
P.H. Zingmark C. Edenius M.O. Karlsson (2004) ArticleTitlePharmacokinetic/Pharmacodynamic models for monoclonal antibody actions on the number of target cells and receptors as measured by FACS Br. J. Clin. Pharmacol. 58 IssueID4 378–389 Occurrence Handle15373930 Occurrence Handle1:CAS:528:DC%2BD2cXpsVShsr0%3D Occurrence Handle10.1111/j.1365-2125.2004.02177.x
P.H. Zingmark M. Ekblom T. Odergren T. Ashwood P. Lyden M.O. Karlsson E.N. Jonsson (2003) ArticleTitlePopulation pharmacokinetics of clomethiazole and its effect on the natural course of sedation in acute stroke patients Br. J. Clin. Pharmacol. 56 173–183 Occurrence Handle12895190 Occurrence Handle1:CAS:528:DC%2BD3sXnsVektbc%3D Occurrence Handle10.1046/j.0306-5251.2003.01850.x
C.A.J Knibbe K.P. Zuideveld J. DeJongh P.F.M Kuks L.P.H.J. Aarts M. Danhof (2002) ArticleTitlePopulation pharmacokinetic and pharmacodynamic modelling of propofol for long-term sedation in critically ill patients: A comparison between propofol 6% and propofol 1% Clin. Pharmacol. Ther. 72 670–684 Occurrence Handle12496748 Occurrence Handle1:CAS:528:DC%2BD3sXlt1Sjsw%3D%3D Occurrence Handle10.1067/mcp.2002.129500
P. Girard T.F. Blaschke H. Kastrissios L.B. Sheiner (2002) ArticleTitleA Markov mixed effect regression model for drug compliance Stat. Med. 17 2313–2333
M.O. Karlsson R.C. Schoemaker B. Kemp A.F. Cohen J.M.A. Gerven ParticleVan B. Tuk C.C. Peck M. Danhof (2000) ArticleTitleA pharmacodynamic Markov mixed-effects model for the effect of temazepam on sleep Clin. Pharmacol. Ther. 68 IssueID2 175–188 Occurrence Handle10976549 Occurrence Handle1:CAS:528:DC%2BD3cXmsFalsr8%3D Occurrence Handle10.1067/mcp.2000.108669
S.L. Beal L.B. Seiner (1992) NONMEM User Guides University of California San Fransisco, CA
E.N. Jonsson M.O. Karlsson (1999) ArticleTitleXpose–an S-PLUS population pharmacokinetic/pharmacodynamic model building aid for NONMEM Comp Meth. Prog. Med. 58 51–64 Occurrence Handle1:STN:280:DyaK1M3htVShsw%3D%3D
E.H. Cox C. Veyrat-Follet S.L. Beal E. Fuesau S. Kenkare L.B. Sheiner (2000) ArticleTitleA population pharmacokinetic-pharmacodynamic analysis of repeated measures time-to-event pharmacodynamic responses: The antiemetic effect of ondansetron J. Pharmacokinet. Biopharm. 27 IssueID6 625–644
J.W. Mandema D.R. Stanski (1996) ArticleTitlePopulation pharmacodynamic model for keterolac analgesia Clin. Pharmacol. Ther. 60 IssueID6 619–635 Occurrence Handle8988064 Occurrence Handle1:CAS:528:DyaK2sXms1eqtg%3D%3D Occurrence Handle10.1016/S0009-9236(96)90210-6
M .O. Karlsson, Jonsson E.N., and P. H. Zingmark. Models for sedation scores in acute stroke patients. In Measurements and Kinetics of in vivo Drug Effects, Advances in Simultaneous Pharmacokinetic/Pharmacodynamic Modelling, 4th International Symposium, 24–27, April 2002, Noordwijkerhout, The Netherlands, 2002
Y. Yano S.L. Beal L.B. Sheiner (2001) ArticleTitleEvaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check J. Pharmacokin Pharmacodyn. 28 IssueID2 171–192 Occurrence Handle1:STN:280:DC%2BD38%2FhtV2htg%3D%3D Occurrence Handle10.1023/A:1011555016423
L.B. Sheiner D.R. Stanski S. Vozeh R.D. Miller J. Ham (1979) ArticleTitleSimultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine Clin Pharmacol. Ther. 25 358–371 Occurrence Handle1:CAS:528:DyaE1MXhsl2qsro%3D
M. Gårdmark L. Brynne M. Hammarlund-Udenaes M.O. Karlsson (1999) ArticleTitleInterchangeability and predicitive performance of empirical tolerance models Clin. Pharmackinet. 36 145–167
M.O. Karlsson S.L. Beal L.B. Sheiner (1995) ArticleTitleThree new residual error models for population PK/PD analyses J. Pharmacokin. Biopharm 23 IssueID6 651–672 Occurrence Handle1:STN:280:DyaK28zivVyntw%3D%3D
H.C. Porchet N.L. Benowitz L.B. Sheiner (1988) ArticleTitlePharmacodynamic model of tolerance: application to nicotine J. Pharmacol. Exp. Ther. 244 231–236 Occurrence Handle3336000 Occurrence Handle1:CAS:528:DyaL1cXht1Kntb0%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zingmark, PH., Kågedal, M. & Karlsson, M.O. Modelling a Spontaneously Reported Side Effect by Use of a Markov Mixed-Effects Model. J Pharmacokinet Pharmacodyn 32, 261–281 (2005). https://doi.org/10.1007/s10928-005-0021-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-005-0021-7