Abstract
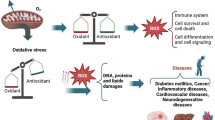
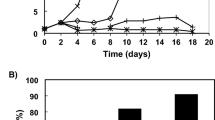
The cells of the red microalga Porphyridium UTEX 637 are encapsulated within a sulfated polysaccharide whose external part (i.e., the soluble fraction) dissolves into the medium. It is thought that the main function of the polysaccharide is to protect the algal cells from the extreme environmental conditions, such as drought and high light, prevailing in their native sea-sand habitat. In this study, we evaluated the antioxidant properties of the water-soluble polysaccharide of Porphyridium sp. by determining the ability of a polysaccharide solution to inhibit: (1) autooxidation of linoleic acid, as determined by the standard thiobarbituric acid (TBA) and ferrous oxidation (FOX) assays; and (2) oxidative damage to 3T3 cells as determined by the dichlorofluorescein (DCFH) assay. In all three assays, the polysaccharide inhibited oxidative damage in a dose-dependent manner. Antioxidant activity was also exhibited by fractions of the polysaccharide obtained by sonication followed by separation on a reverse-phase HPLC with a C8 semi-preparative column. It is suggested that the antioxidant activity of the sulfated polysaccharide protects the alga against reactive oxygen species produced under high solar irradiation, possibly by scavenging the free radicals produced in the cell under stress conditions and transporting them from the cell to the medium.
Similar content being viewed by others
References
Adda M, Merchuk JC, Arad (Malis) S (1986) Effect of nitrate on growth and production of cell wall polysaccharide by the unicellular red alga Porphyridium. Biomass 10: 131–140.
Albertini R, Rindi S, Passi A, Bardoni A, Salvini R, Pallavicini G, De Luca G (1996) The effect of cornea proteoglycans on liposome peroxidation. Arch. Biochem. Biophys. 327: 209–214.
Arad (Malis) S (1988) Production of sulfated polysaccharides from red unicellular algae. In Stadler T, Mollion J, Verdus, MC, Karamanos Y, Morvan H, Christiaen D (eds), Algal Biotechnology, Elsevier Applied Science, London, UK, pp. 65–87.
Arad S, Weinstein Y (2003) Novel lubricants from red microalgae: Interplay between genes and products. Biomedic. (Israel) 1: 32–37.
Bar E, Rise M, Vishkautsan M, Arad (Malis) S (1995) Pigment and structural changes in Chlorella zofingiensis upon light and nitrogen stress. J. Plant Physiol. 46: 527–534.
Ben-Amotz A, Katz A, Avron M (1982) Accumulation of β-carotene in halotolerant algae: Purification and characterization of β-carotene rich globules from Dunaliella bardawil (Chlorophyceae). J. Phycol. 18: 529–537.
Bergman M, Varshavsky L, Gottlieb H, Grossman S (2001) The antioxidant activity of aqueous spinach extract; chemical identification of active fractions. Phytochemistry 58: 143–152.
Bergman M. Perelman A, Dubinsky Z, Grossman, S (2003) Scavenging of reactive oxygen species by a novel glucuronated flavonoid antioxidant isolated and purified from spinach. Phytochemistry 62: 753–762.
Changu X., Guangli Y, Takashi H, Junji T, Hong L (1998) Antioxidative activities of several marine polysaccharides evaluated in a phosphatidylcholine–liposomal suspension and organic solvents. Biosci. Biotechnol. Biochem. 62: 206–209.
Cook NC, Samman S (1996) Flavanoids chemistry, metabolism, cardioprotective effects and dietary sources. Nutr. Biochem. 7: 66–76.
Eteshola E, Gottlieb M, Arad (Malis) S (1996) Dilute solution viscosity of red microalga exopolysaccharide. Chem. Eng. Sci. 51: 1487–1494.
Eteshola E, Karpasas M, Arad (Malis) S, Gottlieb M (1998) Red microalga exopolysaccharides: 2. Study of the rheology, morphology and thermal gelation of aqueous preparations. Acta Polym. 49: 549–556.
Geresh S, Arad (Malis) S (1991) The extracellular polysaccharide of the red microalgae: Chemistry and Rheology. Biores. Technol. 38: 195–201.
Grossman S, Zakut R (1979) Determination of the activity of lipoxygenase (lipoxidase). Methods Biochem. Anal. 25: 303–329.
Huang M-T, Ho C-T, Lee CY (1992) Phenolic compounds in food and their effects on health. II. Antioxidant and cancer prevention. ACS Symposium Series 507, American Chemical Society, Washington, D.C.
Huleihel M, Ishanu V, Tal J, Arad (Malis) S (2001) Antiviral effect of red microalgal polysaccharide on Herpes simplex and Varicella zoster viruses. J. appl. Phycol. 13: 127–134.
Johnson FA, Lewis MJ (1979) Astaxanthin formation by yeast Phaffia rhodozyma. J. gen. Microbiol. 115: 173–189.
Jones RF, Speer HL, Kury W (1963) Studies on the growth of the red alga Porphyridium cruentum. Physiol. Pl. 16: 636–643.
Larson RA (1988) The antioxidants of higher plants. Phytochemistry 27: 969–978.
Lee MJ, Kwon H, Jeong H, Lee JW, Lee SY, Baek S, Surh YJ (2001) Inhibition of lipid peroxidation and oxidative DNA damage by Ganoderma lucidum. Phytother. Res. 15: 245–249.
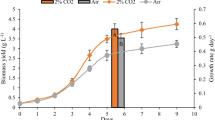
Li SY, Lellouche J, ShabtaiY, Arad (Malis) S (2001) Fixed carbon partitioning in the red microalga Porphyridium sp. (Rhodophyta). J. Phycol. 37: 289–297.
Liu F, Ooi, VEC, Chang ST (1997) Free radical scavenging of mushroom polysaccharide extracts. Life Sci. 60: 763–771.
Lomnitski L (2003), Composition, efficacy and safety of spinach antioxidants. Nutr. Cancer 46: 222–231.
Matsui SM, Muizzudin N, Arad S, Marenus K (2003) Sulfated polysaccharides from red microalgae anti-inflammatory properties in vitro and in vivo. appl. Biochem. Biotechnol. 104 (1): 13–22.
Ramus J, Groves ST (1972) Incorporation of sulfate into the capsular polysaccharide of the red alga Porphyridium. J. Cell Biol. 54: 399–407.
Rice-Evans CA, Burdon R (1993) Free radical lipid interactions and their pathological consequences. Prog. Lipid Res. 32: 71–110.
Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chem W, Knapp W, Zlabinger GJ (1992) A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescein diacetate. J. Immunol. Methods 156: 39–45.
Ruperez P, Ahrazem Q, Leal JA (2002) Potential antioxidant activity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 50: 840–845.
Savins JC (1978) Oil recovery process employing thickened aqueous driving fluid. Patent No. 4,079,544. United States Patent.
Shrestha RP, Weinstein Y, Bar–Zvi D, Arad (Malis) S (2004) A non–covalently bound cell wall glycoprotein of the red microalga Porphyridium sp. J. Phycol. 40: 568–580.
Thepenier C, Gudin C, Thomas D (1985) Immobilization of Porphyridium cruentum in polyurethane foam for the production of polysaccharide. Biomass 7: 225–240.
Tsiapali E, Whaley S, Kalbfleisch J, Ensley HE, Browder IW, Williams DL (2001) Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Rad. Biol. Med. 30: 393–402.
Wasowicz W, Neve J, Peretz A (1993) Optimized steps in fluorometric determination of thiobarbituric acid–reactive substances in serum: Importance of extraction pH and influence of sample preservation and storage. Clin. Chem. 39: 2522–2526.
Wolf SP (1994) Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurment of hydroperoxides. In: Packer L (ed.), Methods in Enzymology, Academic Press Inc., pp. 182–189.
Xue CH, Fang Y, Lin H, Chen L, Li ZJ, Deng D, Lu CX (2000) Chemical characters and antioxidant properties of sulfated polysaccharides from Laminaria japonica. J. appl. Phycol. 13: 1–5.
Yaron A, Dvir I, Maislos M, Mokady S, Arad (Malis) S (1995) The red microalga Rhodella reticulata as a source of dietary ω-3 highly unsaturated fatty acid- eicosapentaenoic acid. In Charalambous G (ed.). Food Flavors: Generation, Analysis and Process Influence. Elsevier Science B.V. 665–674.
Zaho X, Xue CH, Li ZJ, Cai YP, Liu HY, Qi HT (2004) Antioxidant and hepatoprotective activities of low molecular weight sulfated polysaccharide from Laminaria japonica. J. appl. Phycol. 16: 111–115.
Zhang Q, Yu P, Li Z, Zhang H, Xu Z, Li P (2003) Antioxidant activities of sulfated polysaccharide fractions from Porphyra haitanesis. J. appl. Phycol. 15: 305–310.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tannin-Spitz, T., Bergman, M., van-Moppes, D. et al. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J Appl Phycol 17, 215–222 (2005). https://doi.org/10.1007/s10811-005-0679-7
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10811-005-0679-7