Abstract
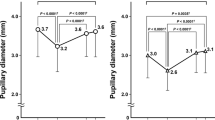
The aim of this study was to evaluate pupillary response to light stimulation in patients with different stages of glaucoma using computerized pupillometry. We conducted a retrospective study on a group of 44 glaucoma patients who had undergone complete ophthalmological examination, visual field test (Humphrey SITA Standard 24-2) and monocular dynamic pupillometry (MonCV3 Metrovision). Eyes were classified into stages of glaucoma according to visual field damage using the Glaucoma Staging System 2. A group of 18 healthy subjects, homogeneous for age and sex with glaucoma patients, was used as a control. The following parameters were considered—latency and duration of contraction and dilatation; initial, minimum, maximum, and mean pupil diameter; amplitude of contraction; contraction and dilatation speed; and percent pupil contraction (PPC). PPC and pupil contraction speed and minimum diameter showed covariate correlation with the stages of glaucoma. The control group significantly differed from the stage 3 group in terms of PPC and from the stage 4 group in terms of minimum diameter. There were significant differences between the stage 5 group and stage 1, 2, 3 and control groups. Ordinal logistic regression showed a correlation between pupil contraction speed, minimum diameter, PPC, initial diameter and the stage of glaucoma. The study showed that glaucoma damage is associated with altered values of pupillary response to light. This event may be the consequence of the progressive loss of retinal ganglion cells and their axons induced by glaucoma.




Similar content being viewed by others
References
Kawasaki A (1999) Physiology, assessment, and disorders of the pupil. Curr Opin Ophthalmol 10:394–400
Levatin P, Prasloski PF, Collen MF (1973) The swinging flashlight test in multiphasic screening for eye disease. Can J Ophthalmol 8(2):356–360
Kaback MB, Burde RM, Becker B (1976) Relative afferent pupillary defect in glaucoma. Am J Ophthalmol 81:462–468
Garaci FG, Bolacchi F, Cerulli A et al (2009) Optic nerve and optic radiation neurodegeneration in patients with glaucoma: in vivo analysis with 3-T diffusion-tensor MR imaging. Radiology 252(2):496–501
Bolacchi F, Garaci FG, Martucci A et al (2012) Differences between proximal versus distal intraorbital optic nerve diffusion tensor magnetic resonance imaging properties in glaucoma patients. Invest Ophthalmol Vis Sci 53(7):4191–4196
Resnikoff S, Pascolini D, Etya’ale D et al (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82:844–851
Cedrone C, Mancino R, Cerulli A, Cesareo M, Nucci C (2008) Epidemiology of primary glaucoma: prevalence, incidence, and blinding effects. Prog Brain Res 173:3–14
Grozdanic SD, Betts DM, Sakaguchi DS, Kwon YH, Kardon RH, Sonea IM (2003) Temporary elevation of the intraocular pressure by cauterization of vortex and episcleral veins in rats causes functional deficits in the retina and optic nerve. Exp Eye Res 77(1):27–33
Lowenstein O, Loewenfeld I (1958) Electronic pupillography. Arch Ophthalmol 59:352–363
Chen Y, Wyatt HJ, Swanson WH (2005) Pupillary evaluation of retinal asymmetry: development and initial testing of a technique. Vision Res 45(19):2549–2563
Link B, Jünemann A, Rix R et al (2006) Pupillographic measurements with pattern stimulation: the pupil’s response in normal subjects and first measurements in glaucoma patients. Invest Ophthalmol Vis Sci 47(11):4947–4955
Scuderi G, Papale A, Nucci C, Cerulli L (1995) Retinal involvement in pigment dispersion syndrome. Int Ophthalmol 19(6):375–378
Chen Y, Kardon RH (2013) Studying the effect of iris mechanics on the pupillary light reflex using brimonidine-induced anisocoria. Invest Ophthalmol Vis Sci 54(4):2951–2958
Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR (1998) The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology 105(4):733–739
Lamparter J, Russell RA, Schulze A, Schuff AC, Pfeiffer N, Hoffmann EM (2012) Structure-function relationship between FDF, FDT, SAP, and scanning laser ophthalmoscopy in glaucoma patients. Invest Ophthalmol Vis Sci 53(12):7553–7559
Brusini P, Filacorda S (2006) Enhanced glaucoma staging system (GSS 2) for classifying functional damage in glaucoma. J Glaucoma 15(1):40–46
Hennessy AL, Katz J, Ramakrishnan R et al (2011) The utility of relative afferent pupillary defect as a screening tool for glaucoma: prospective examination of a large population-based study in a south Indian population. Br J Ophthalmol 95(9):1203–1206
Kalaboukhova L, Fridhammar V, Lindblom B (2007) Relative afferent papillary defect in glaucoma: a pupillometric study. Acta Ophthalmol Scand 85:519–525
Nucci C, Bari M, Spanò A et al (2008) Potential roles of (endo) cannabinoids in the treatment of glaucoma: from intraocular pressure control to neuroprotection. Prog Brain Res 173:451–464
Russo R, Berliocchi L, Adornetto A et al (2011) Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis 14(2):e144
Nucci C, Morrone L, Rombolà L, Nisticò R, Piccirilli S, Cerulli L (2003) Multifaceted roles of nitric oxide in the lateral geniculate nucleus: from visual signal transduction to neuronal apoptosis. Toxicol Lett 139(2–3):163–173
Pickard GE, Sollars PJ (2011) Intrinsically photosensitive retinal ganglion cells. Rev Physiol Biochem Pharmacol 162:59–90
Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW (2003) Diminished pupillary light reflex at high irradiances in melanopsin knockout mice. Science 299:245–247
Do MT, Yau KW (2010) Intrinsically photosensitive retinal ganglion cells. Physiol Rev 90(4):1547–1581
Jakobs TC, Libby RT, Ben Y, John SW, Masland RH (2005) Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2 J mice. J Cell Biol 171:313–325
Drouyer E, Dkhissi-Benyahya O, Chiquet C et al (2008) Glaucoma alters the circadian timing system. PLoS One 3(12):e3931
Wang HZ, Lu QJ, Wang NL (2008) Loss of melanopsin-containing retinal ganglion cells in a rat glaucoma model. Chin Med J 121:1015–1019
de Zavalia N, Plano SA, Fernandez DC et al (2011) Effect of experimental glaucoma on the non-image forming visual system. J Neurochem 117:904–914
Schmidt TM, Do MT, Dacey D, Lucas R, Hattar S, Matynia A (2011) Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci 31(45):16094–16101
Acknowledgments
No author has any financial or commercial interests in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martucci, A., Cesareo, M., Napoli, D. et al. Evaluation of pupillary response to light in patients with glaucoma: a study using computerized pupillometry. Int Ophthalmol 34, 1241–1247 (2014). https://doi.org/10.1007/s10792-014-9920-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-014-9920-1