Abstract
Pulmonary hypertension and concomitant right ventricular failure present a diagnostic and therapeutic challenge in the intensive care unit and have been associated with a high mortality. Significant co-morbidities and hemodynamic instability are often present, and routine critical care unit resuscitation may worsen hemodynamics and limit the chances of survival in patients with an already underlying poor prognosis. Right ventricular failure results from structural or functional processes that limit the right ventricle’s ability to maintain adequate cardiac output. It is commonly seen as the result of left heart failure, acute pulmonary embolism, progression or decompensation of pulmonary hypertension, sepsis, acute lung injury, or in the perioperative setting. Prompt recognition of the underlying cause and institution of treatment with a thorough understanding of the elements necessary to optimize preload, cardiac contractility, enhance systemic arterial perfusion, and reduce right ventricular afterload are of paramount importance. Moreover, the emergence of previously uncommon entities in patients with pulmonary hypertension (pregnancy, sepsis, liver disease, etc.) and the availability of modern devices to provide support pose additional challenges that must be addressed with an in-depth knowledge of this disease.
Similar content being viewed by others
Introduction
The understanding of the function of the right ventricle (RV) in health and disease is an understudied field that has evolved considerably over the last century. The RV, once considered a passive conduit of blood and a bystander in disease, is now recognized to play an important role in determining the outcome of many patients admitted to the intensive care unit (ICU) [1–3]. Acute dysfunction of the RV, irrespective of baseline pulmonary vascular resistance (PVR), has been a well-recognized entity in ICU patients with septic shock and acute respiratory distress syndrome (ARDS) [4, 5]. The decompensation of the RV function is detrimental to survival, especially in patients with pulmonary hypertension (PH) who often have compromised RV function at baseline [6]. Despite the better understanding and available therapies for PH, the mortality of patients admitted to the ICU with decompensated PH and right ventricular failure (RVF) remains unacceptably high [7–10]. This review will describe the epidemiology, evaluation, and management of patients requiring hospitalization and ICU admission for decompensated PH and RVF.
Epidemiology
The true prevalence of PH is unknown, likely due to the variable etiologies which have been classified into groups by the World Health Organization (WHO) (Table 1) [11]. Recent literature estimates the prevalence of group 1 (PAH) which is around 15 per million people [12, 13]. Left heart disease is the most common cause of PH and comprises group 2 with an estimated 25–100 % of patients with left heart disease having PH [14]. Chronic obstructive pulmonary disease (COPD) is the most common cause of group 3 PH, with prevalence varying with disease severity. Over 90 % of patients with severe COPD have a mean pulmonary artery pressure (mPAP) > 20 mmHg, and 3–5 % have mPAP > 35–40 mmHg [15, 16]. Chronic thromboembolic pulmonary hypertension (CTEPH), group 4, occurs in up to 3.8 % of patients suffering from an acute pulmonary embolism (PE) with an estimated prevalence of 3.2 cases per million adults [15–17]. Group 5 includes patients with PH from multifactorial mechanisms with an unknown prevalence. As a whole, groups 2–5 are more common than group 1 and can progress to RVF conveying a higher mortality risk [18–21].
Right ventricular failure
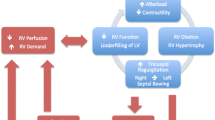
RVF results from a structural or functional process that limits the right ventricle’s ability to effectively pump blood through the pulmonary circulation to maintain adequate filling of the left ventricle (LV) and cardiac output (CO) [9, 22]. These processes cause derangements in RV preload, contractility, or afterload (Table 2). The most frequent causes of decompensation are infection, anemia, trauma, surgery, unplanned modification or withdrawal of pulmonary vasodilator therapy, unplanned withdrawal of diuretics, cardiac arrhythmias, pregnancy, and PE. However, up to 48 % of cases have no apparent causative factor and mortality is very high, ranging from 32 to 61 % [10].
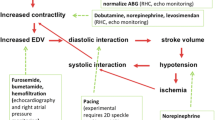
The RV is more effective at adapting to volume overload than it is to pressure overload, and the outcome of RVF depends on the underlying cause. A gradual increase in RV afterload leads to chronic adaptation of the RV enabling it to tolerate a significant elevation in pulmonary artery pressures (PAP), whereas the RV without pre-existing hypertrophy will be unable to generate a systolic PAP above 50–60 mmHg [7–10, 23]. RVF secondary to PE has a much better prognosis compared to decompensated PH in a patient with underlying connective tissue disease (CTD) [10]. The management of the patient with RVF is complex and should include the investigation of the underlying causes, appropriate routine ICU care, and hemodynamic optimization (Fig. 1).
Treatment of acute right ventricular (RV) failure in the intensive care unit (ICU). In addition to routine ICU care, treatment of RV failure consists of treating the underlying cause and optimizing hemodynamics in systematic approach. PE pulmonary embolism, PH pulmonary hypertension, LV left ventricle, CTPH chronic thromboembolic pulmonary hypertension, VAD ventricular assist device, ECMO extracorporeal membrane oxygenation
Initial evaluation of RVF
The initial evaluation of any patient in the ICU should focus on promptly establishing the cause of decompensation and identifying reversible conditions. Owing to the increasing awareness of PH, most patients suspected of having RVF from PH have a pre-existing diagnosis [23]. However, physical examination findings may be helpful in the unknown patient, and, although no specific biomarkers for RVF exist, several serum chemistries, cardiac enzymes, imaging, and diagnostic tests aid in the diagnosis and prognosis.
Physical examination
Physical examination, especially in the early stages, is neither sensitive nor specific. Patients can present with tachycardia, tachypnea, hypotension, hypoxia, anxiety, cyanosis, and facial plethora. Cardiac examination is characterized by an elevated jugular venous pulse with a large “v” wave, a prominent pulmonary component of the second heart sound (P2), a palpable RV heave, and a holosystolic tricuspid regurgitant murmur along the left lower sternal border that increases during inspiration. The height of the jugular venous distention and the quality of the venous wave pattern should be assessed (elevated a vs. v wave). The distance from the sternal angle to the top of the waveform is measured in centimeters, and by convention, the distance between the right atrium (RA) and the sternal angle is added (approximately 5 cm) [24]. Auscultation of the lungs is usually unremarkable unless an underlying condition exists (COPD, pulmonary fibrosis, ARDS). Patients often have tender, palpable hepatomegaly, ascites, and peripheral edema. Cyanosis and digital clubbing may be seen, especially in patients with chronic hypoxemia. Findings of underlying conditions such as CTD (e.g., telangiectasia, sclerodactyly, and malar rash) may also be apparent [25, 26].
Laboratory and ancillary examinations
Although no specific test exists to diagnose RVF, several routine and disease-specific biochemical parameters aid in the diagnosis, management, and prognostication of patients with decompensated PH and RVF (Table 3). Liver function tests may be abnormal as a result of underlying liver disease or due to hepatic congestion. Patients with Na ≤ 136 mEq/L have more symptoms, markers for RV dysfunction, and higher hospitalization and mortality rates (HR = 10.16) than their counterparts with normal sodium levels [7, 27]. Elevated serum creatinine level is associated with a worse hemodynamic profile and increased mortality [7, 28]. Elevated C-reactive protein (CRP), common in infection and inflammation, is associated with an increased mortality [29, 30].
Cardiac enzymes, although not specific for RVF, can be elevated in settings overstretching and ischemia of the RV. B-type natriuretic peptide (BNP) is closely related to the functional impairment of PAH patients and parallels the extent of pulmonary hemodynamic changes and RVF [31]. It can provide prognostic information in patient with stable PH as well as in decompensated patients admitted to the ICU [32–37]. High-sensitivity troponin T levels have been associated with higher risk of death and hospitalization in patients with PH [38–40]. Moreover, patients presenting with acute PE and elevated troponin or BNP levels have a higher risk of adverse outcomes and mortality than those with normal levels [36, 37, 41].
Electrocardiography (ECG) is specific (83–95 %) but not sensitive (18–43 %) enough for the diagnosis of right ventricular hypertrophy (RVH) [42–44]. However, ECG parameters reflective of physiologic and anatomic abnormalities in the RV are significant predictors of mortality in patients with PAH. These include p-wave amplitude >0.25 mV in lead II, presence of qR in V1, and the WHO RVH criteria (Fig. 2) [45]. In addition, the ECG may reveal signs of RV ischemia or infarct.
Imaging
Radiographic examinations of the chest including chest X-ray and CT scan lack sensitivity or specificity in the diagnosis of early RV decompensation or failure, and their use is limited. Nonetheless, they can play an important role in defining an underlying pulmonary disease (pneumonia, pulmonary fibrosis, PE, etc.) [46]. Cardiac magnetic resonance imaging is a very effective noninvasive method to assess RV function, but is rarely used in the management of critically ill patients due to logistical issues [47].
Diagnostic strategies
Since electrocardiography and different biomarkers are not sensitive for the diagnoses of RVF in the ICU, the most reliable methods of diagnosis and monitoring of treatment response in the ICU are echocardiography (transthoracic and/or transesophageal) and the pulmonary artery catheter (PAC).
Echocardiography
Bedside echocardiography has a pivotal role in the critically ill patient with decompensated PH and RVF, as it can provide information regarding the morphology and function of the RV, estimate RA and RV pressures, and identify cardiac causes of PH (Table 4) [48, 49].
Disease states that cause RV volume or pressure overload will lead to dilation and eventual hypertrophy of the RV. Findings of significant PH on echocardiography include: inferior vena cava dilatation, RA enlargement, RV enlargement and/or hypertrophy, decreased RV function, intraventricular septal flattening (D-shaped LV), and tricuspid regurgitation (TR) (Figs. 3, 4, 5). The TR jet or the pulmonary regurgitation jet velocities, in conjunction with an estimated RA pressure, are used to calculate the right ventricular systolic pressure (RVSP). RVSP correlates well with systolic PAP in the absence of pulmonary stenosis and can diagnose PH with a sensitivity of 83 % and specificity of 72 % (Fig. 5) [50, 51]. However, about 10–25 % of patients will have an insufficient spectral Doppler profile of the TR jet to measure the RV to RA pressure gradient; in these instances, the presence of right heart chamber enlargement or septal flattening suggests elevated right heart pressures [52, 53].
Two-dimensional echocardiography (parasternal short-axis view) showing ventricular interdependence. Normally, LV end-diastolic pressure is greater than RV end-diastolic pressure, and the septum bows toward the RV during diastole (a, b). In patients with pulmonary hypertension and RV failure, RV end-diastolic pressure exceeds that of the LV and the septum bows toward the LV during diastole forming a “D”-shaped pattern and impaired LV filling. Also, notice red arrow pointing to pericardial effusion (c). The combination of high RV systolic pressure and decreased LV filling may lead to near obliteration of the LV at end-systole (d)
Right ventricular systolic pressure (RVSP) estimation using the tricuspid regurgitation jet V max with CW Doppler. Bernoulli equation: pressure gradient = 4 × V max 2. RAP is estimated using the size and collapsibility (during inspiration) of the IVC. V max maximum velocity, CW continuous wave, RAP right atrial pressure
The severity of symptoms in patients with PH is strongly associated with RV function. This can be evaluated by echocardiography using multiple parameters including the fractional area change (FAC), RV free-wall longitudinal systolic tissue velocity(s’), tricuspid annular plane systolic excursion (TAPSE) (Fig. 6), RV myocardial performance index (MPI or Tei index), isovolumic contraction velocity (IVCv), RV strain, and 3D RV ejection fraction [54–57].
Echocardiography provides prognostic information in patient with PH and RVF (Table 5). Several echocardiographic parameters of RV dysfunction predict worse outcomes including an increased RV diameter, a decreased TAPSE, an elevated Tei, an increased RA area, a decreased isovolumic contraction velocity (IVCv), and alterations in RV free-wall strain [58–65]. In addition, the presence and severity of a pericardial effusion, theoretically caused by elevated RA pressures due to RV dysfunction leading to impaired lymphatic drainage through the thoracic duct, are a strong predictor of mortality (Fig. 4) [63, 66, 67].
Pulmonary artery catheter
Accurate and complete invasive assessment of pulmonary hemodynamics is essential in the evaluation of patients with RVF, especially in those with RVF due to PH, as some hemodynamic values are predictors of survival [68–71]. In patients with newly diagnosed primary PH, the NIH registry showed that mortality was closely associated with an increased mean RA pressure, an increased mPAP, and a decreased cardiac index. An increase in mPAP from <55 mmHg to ≥85 mmHg correlated with a decrease in median survival from 48 months to 12 months, an increase in RA pressure from <10 mm Hg to ≥20 mm Hg was associated with a decrease in median survival from 46 months to 1 month, and an increase in cardiac index from <2.0 L/min/m2 to ≥4.0 L/min/m2 correlated with an increase in survival time from 17 months to 43 months. Although these parameters have prognostic implications in chronic disease, their significance in acute decompensated PH or RVF has not been established [68].
In the critically ill patients, the hemodynamic values obtained when placing a PAC can help to assess the response to pharmacologic agents and aid in their titration to meet specific endpoints. When evaluating hemodynamic variables in a patient with RVF, the clinician must keep in mind that the mPAP may decrease as the RV function worsens. While patients who respond to the acute vasoreactivity test have excellent prognosis (95 % survival at 5 years), while on calcium channel blockers, the utility of this test in the critical care setting is very limited [72, 73].
The placement of a PAC, albeit invasive, is a relatively safe procedure, especially when performed by experienced operators. A large cohort study reviewed, retrospectively and prospectively, the placement of 7218 PACs at 20 major pulmonary vascular centers over a 5-year period [74]. The overall number of serious adverse events was 76 (1.1, 95 CI 0.8–1.3 %). The most frequent complications were related to venous access (e.g., hematoma, pneumothorax), arrhythmias, and hypotension related to vagal reactions or pulmonary vasoreactivity testing. Only four fatal events were recorded, resulting in an overall procedure-related mortality of 0.055 %. Similar results have been published in more recent but smaller cohorts [74, 75]. Although the evidence does not support the routine placement of PACs in the overall critical care population, this intervention has not been studied in patients with acute RVF and its use is advocated by most experts to monitor hemodynamic parameters and mixed venous oxygen saturation (SvO2) [9, 23, 76, 77].
Hemodynamic management
The hemodynamic optimization of patients with RVF is complex and encompasses the precise manipulation of different variables to improve systemic perfusion including optimization of preload, enhancement of cardiac contractility, RV afterload reduction, and maintenance of systemic perfusion pressure.
Preload Optimization
The optimization of right-sided filling pressures is crucial in the management of RVF as both hypovolemic and hypervolemic states have deleterious effects leading to decreased CO. In hypovolemic patients, fluid loading produces a 30 % mean increase in right ventricular end-diastolic volume index (RVEDVI) and a 17 % increase in LV end-diastolic volume index (LVEDVI) resulting in an enhanced stroke volume index (SVI) [78]. Similar response to fluid loading has been described in patients with RVF caused by massive PE [79]. The clinician must exert caution because excessive fluid administration in patients with increased PVR can have adverse effects that are explained by the phenomenon of ventricular interdependence (displacement of the interventricular septum toward the LV leading to decreased LV preload as seen in Fig. 4) and increased RV free-wall tension that result in increased myocardial oxygen consumption and decreased perfusion [80–83]. Therefore, liberal volume administration should be discouraged.
Most cases of RVF are associated with volume overload requiring the administration of diuretics or ultrafiltration to achieve a negative fluid balance. However, excessive volume removal can also be detrimental by reducing the already impaired CO. Although the optimal filling pressures vary considerably between individual patients, preload should be kept at a goal between 8 and 12 mm Hg and subsequently adjusted to optimize cardiac output [22]. In summary, the clinician will need to closely monitor the effect of fluid administration or removal on filling pressures, CO, and perfusion parameters.
Enhancement of cardiac contractility
The enhancement of RV contractility with the addition of inotropic agents (Table 6) is important in the management of RVF.
Dobutamine, the most commonly used agent, has very dose-specific hemodynamic effects. At low doses (up to 5 μg/Kg/min), it produces increased cardiac contractility and decreased systemic vascular resistance (SVR) and PVR. At higher doses, it is associated with tachycardia and premature ventricular contractions without further reduction in the PVR [84]. In animal studies, doses > 10 μg/Kg/min were associated with increased PVR and hypotension [85]. The clinician must be aware of the latter complication that often necessitates vasopressors. Dobutamine is more effective than norepinephrine at improving CO, but it can increase the shunt fraction (venous admixture) affecting oxygenation (PaO2). Simultaneous administration of dobutamine and inhaled nitric oxide can improve cardiac output without impairing oxygenation [86–88].
Milrinone produces a significant improvement in RV contractility, decrease in PVR, and improvement in LV filling [89, 90]. However, because of its vasodilator properties, it can cause or worsen preexisting hypotension [91]. Several studies report that inhaled delivery is well tolerated and produces similar effects in PAP and PVR with a higher SVR and less hypotension than the intravenous form [92–94]. In addition, the combination of milrinone and iNO has been associated with a more pronounced decrease in PAP than either agent alone [95].
Levosimendan has inotropic and vasodilator properties without increasing oxygen demand [96]. It increases right ventricular contractility and produces pulmonary vasodilation in patients with ARDS and RVF caused by PE [97, 98]. In canine models, levosimendan had similar inotropic effects and stronger pulmonary vasodilatory effects than dobutamine [99]. In comparison with milrinone, levosimendan exerts a positive inotropic effect with a smaller increase in myocardial oxygen consumption [100]. In spite of these promising results, levosimendan has not been fully investigated in patients with RVF and its use can be complicated by arrhythmias and a low SVR leading to hypotension [98, 101–103]. Therefore, more evidence is needed before levosimendan can be widely used for this indication.
Vasopressor agents to restore systemic blood pressure
Maintaining adequate systemic arterial pressure has a dual importance in patients with RVF as it allows the organs to maintain autoregulation of perfusion and preserves blood flow to the right coronary artery (RCA) territory which is perfused throughout the cardiac cycle. The reduced RCA driving pressure due to lower aortic root pressure and/or higher RV pressure is detrimental to RV coronary perfusion. Therefore, increasing the aortic root pressure and SVR by using vasopressors in the setting of increased RV afterload will improve perfusion to the RCA territory [81, 104]. The ideal vasopressor agent should increase systemic arterial pressure (SVR) with minimal effect on the PVR (reduce the PVR/SVR ratio) and improve contractility of the RV. The clinician must exert caution because these drugs (Table 7) can increase PVR and cause unwanted effects.
Norepinephrine predominantly produces systemic vasopressor effects through the α1 receptors. Doses of 0.5 μg/Kg/min do not cause a significant increase in PAP, whereas doses of 10 μg/Kg/min are sufficient to produce a 50 % increase in PVR. However, such high doses are not typical in the management of ICU patients [86, 105]. Norepinephrine reduces the PVR/SVR ratio in patients with chronic PH and can improve myocardial oxygen delivery to the RV in septic patients with RVF [106, 107]. The stimulation of β1 receptors improves the CO and the RV/PA coupling which is a measure of the efficiency of transmission of energy from RV to PA [86, 107, 108].
Dopamine increases CO and SVR. It exerts a pulmonary vasoconstrictor effect that can increase the PVR/SVR ratio leading to a decrease in the left to right shunt seen in infants with patent ductus arteriosus [109, 110]. Animal studies show that doses ≤10 µg/Kg/min increase CO leading to an increase in PAP without increasing the PVR [111, 112]. Nonetheless, dopamine has been associated with tachycardia in patients with PH, increased risk of arrhythmias in patients with septic shock, and increased mortality in patients with cardiogenic shock [113, 114].
Epinephrine has a potent vasoconstrictor effect due to its α1 activity. In animal models, epinephrine at doses from 0.2 to 3.2 µg/Kg/min was superior to dopamine in decreasing the PVR/SVR ratio [115]. After epinephrine administration, patients with RVF caused by septic shock experienced improved RV contractility, CO and mPAP without significantly affecting the PVR [116].
Phenylephrine has vasopressor but no inotropic activity. It has the ability to improve perfusion to the RCA because of the elevation of SVR [81, 117]. However, due to its effect of increasing the PVR, it can significantly impair RV function and decrease CO [106, 108].
Vasopressin produces systemic vasoconstriction while relatively sparing the pulmonary circulation; such combined effects produce a desirable decrease in the PVR/SVR ratio [118–123]. Vasopressin has been used in pediatric and adult settings as a rescue agent in the management of PH and RVF [120, 124–126]. However, at high doses (>0.4 U/min), vasopressin can cause bradycardia and decrease in RV contractility and CO likely related to a decrease in coronary blood flow [127–130].
In summary, norepinephrine is the preferred agent given its effects on SVR, PVR, and improvement in RV/PA coupling. Vasopressin at low doses, dopamine, and norepinephrine are reasonable alternatives. Phenylephrine must be used with caution given the isolated α1 effect that will increase systemic perfusion but will also increase PVR, potentially worsening RV function.
RV afterload reduction
Reduction in PVR is one of the most important components in the management of RVF because of the particular sensitivity of the RV to afterload changes. The pharmacologic agents (Table 8) to reduce PVR must be used with caution as they have the potential to cause hypotension.
Inhaled nitric oxide (iNO) is a very potent pulmonary vasodilator. Once inhaled, it diffuses across the alveolar capillary membrane into the smooth muscle of the pulmonary vessels and is rapidly inactivated by hemoglobin [131, 132]. iNO decreases PVR, has a neutral effect on SVR, and increases CO [133, 134]. In patients with RVF secondary to ARDS, iNO decreases PAP, increases PaO2 by improving the ventilation perfusion relationship, and may decrease inflammatory cytokine production in the lungs [135–137]. iNO improves oxygenation with lower doses than those required to decrease mPAP, but it can have the opposite effect on oxygenation at higher doses [138]. Small studies have shown that iNO produces hemodynamic improvement in different settings of RVF including those caused by RV infarct, acute PE, post-surgical, post-left ventricular assist device (LVAD) implantation, and cardiac transplantation [139–155]. Complications encountered with the use of iNO include: accumulation of potentially toxic reactive metabolites, rebound PH, and rarely methemoglobinemia [156–161]. Despite reducing afterload and improving hypoxemia in RVF and ARDS, there is no demonstrated survival benefit [162–164]. Combination of iNO and prostacyclin derivatives has been reported with success in the perioperative management of portopulmonary hypertension (PoPH) and in RVF after high-risk cardiac surgery, LVAD insertion, and pulmonary endarterectomy [165–169].
The phosphodiesterase-5 (PDE5) inhibitors prevent the hydrolysis of cyclic guanosine monophosphate (cGMP), producing vasodilatory and antiproliferative effects in the pulmonary vasculature [170]. Sildenafil produces an acute decline in mPAP and PVR associated with an increase in CO. The vasodilatory effect of sildenafil is comparable to the effect of iNO with the added benefit of maintaining the pulmonary capillary wedge pressure (PCWP) and producing systemic vasodilatory effects [171–173]. Sildenafil has been used with success in patients with RVF at induction of anesthesia, during cardiac surgery, after LVAD placement, after cardiac transplant, and as an adjunct in the weaning of iNO to prevent rebound PH [174–181].
Prostacyclin derivatives are potent systemic and pulmonary vasodilators. These drugs act on the prostacyclin receptor (present in platelets and endothelial cells) that produces an increase in cyclic adenosine monophosphate (cAMP), resulting in inhibition of platelet aggregation, relaxation of smooth muscle, and vasodilation of the pulmonary arteries [182]. Prostacyclin derivatives decrease PVR and increase CO and exercise capacity in patients with PAH and have been used successfully in the perioperative setting in cardiac surgery and thoracic transplant [183–194]. In the setting of life-threatening PE, inhaled aerosolized prostacyclin was associated with a transient improvement in pulmonary hemodynamics and gas exchange [195]. In ARDS, inhaled prostacyclins have been associated with important physiologic benefits (improved hypoxemia, lower PAP, and improved RV function and CO) with no systemic hemodynamic effects given the aerosolized alveolar delivery. Unfortunately, there is no evidence supporting outcome benefits and, although controversial, its use should be reserved as a rescue therapy in refractory hypoxemia associated with ARDS [196–198].
Although the other available vasodilator agents including endothelin receptor antagonists (ERAs) and soluble guanylate cyclase stimulator (riociguat) are effective in PH, their use is not recommended in the setting of RVF. The ERAs have long half-lives and are associated with liver toxicity [199, 200]. Riociguat is a potent pulmonary vasodilator that produces significant systemic vasodilation and hypotension [201].
Intensive care unit supportive management
In addition to routine ICU care (nutrition, prophylaxis, etc.), the specific goals for care provided in the ICU are aimed at managing the factors that would further impair the function of the failing RV. The management of hypoxemia, acidemia, increased intrathoracic pressures caused by mechanical ventilation, and the treatment of cardiac arrhythmias are extremely important.
Hypoxemia and acidemia
The management of the patient with RVF must address low oxygen saturation and acidemia as both increase mPAP and PVR with synergistic effects [202–205]. An oxygen saturation level ≥92 % has been proposed as an ideal target by experts [9, 22]. While acidemia increases the sensitivity of the pulmonary vasculature to hypoxemia, alkalemia decreases such sensitivity and produces pulmonary vasodilation [202]. Therefore, the clinician must strive for a PCO2 and pH as close to normal as possible.
Mechanical ventilation
Mechanical ventilation, while often necessary in the management of patient with RVF, has the potential to produce unfavorable hemodynamic effects. The skillful adjustment of the ventilator to improve oxygenation and acidemia while minimizing the effects of increased intrathoracic pressures on the cardiovascular system becomes a priority.
Positive pressure ventilation causes decreased venous return, decreased RV stroke volume, distention of alveoli and compression of alveolar blood vessels, and increased PVR. Lung volumes near the functional residual capacity (FRC) minimally affect the PVR, whereas atelectasis and overdistention increase it (Fig. 7) [206–208]. While the patient with normal RV function may tolerate these changes relatively well, the consequences can be severe in patients with impending RVF. Therefore, the ventilatory strategy in the patient with RVF must strive to achieve normoxia using conservative tidal volumes and PEEP to avoid atelectasis, overdistention, increased PVR, and elevated intrathoracic pressures.
Relationship of lung volumes and PVR. At volumes near FRC, the PVR is minimally affected. Atelectasis compresses extra-alveolar blood vessels leading to increased PVR. At high lung volumes, alveolar overdistension compresses intra-alveolar blood vessels resulting in increased PVR. RV residual volume, FRC functional residual capacity, TLC total lung capacity
Although protective lung strategies are typically associated with respiratory acidosis, they confer a mortality benefit in ARDS that may be partially related to the lower incidence of RVF seen with lower tidal volumes and plateau pressures [209, 210]. Unfortunately, acute cor pulmonale is present in 20–25 % of patients with ARDS ventilated with protective lung strategies. Although this represents a decrease in the incidence of RVF from the pre-protective lung strategies era, it is still associated with poor clinical outcomes [210–215].
Prone positioning is associated with a significant decrease in airway pressures, PaCO2, and improvement in echocardiographic parameters of RV pressure overload [216]. Moreover, it provides a mortality benefit in patients with severe ARDS [217].
High-frequency oscillatory ventilation (HFOV) is associated with unfavorable hemodynamic effects including an increase in central venous pressure, PCWP, and decrease in CO [218]. In patients with ARDS, HFOV can worsen RV function and does not provide a mortality benefit when compared with conventional protective lung strategies [219–221].
Rhythm control
Cardiac arrhythmias can be a cause or a complication of RVF in patients admitted to the ICU. Atrial flutter and fibrillation are the most common arrhythmias in this population, whereas bradyarrhythmias and ventricular arrhythmias are rare except in the setting of cardiac arrest [222]. The RV is very sensitive to abnormalities in cardiac rhythm and synchrony. In RVF, augmented RA contraction and intact atrioventricular (AV) synchrony are important determinants of CO. The augmented RA contractility constitutes a compensatory response to RV dysfunction [223]. Restoration of AV synchrony produced positive hemodynamic results in patients with RV infarct and congenital heart disease [224–229]. AV pacing in patients with right bundle-branch block and RV dysfunction augments RV and systemic performance [230]. Although there are no large-scale studies to support resynchronization therapy in RVF, this intervention could be considered as part of the management.
Mechanical circulatory support
Mechanical circulatory support is typically reserved for patients with persistent RVF in spite of medical interventions and used as a bridge to heart, lung, or dual heart–lung transplantation. The currently available mechanical circulatory support modalities include the different ventricular assist devices and extracorporeal membrane oxygenation (ECMO).
The mechanical assist devices are available as left, right, or biventricular (LVAD, RVAD, or BiVAD, respectively). LVADs have been used with success in patients with PH and RVF caused by left heart dysfunction with the goal to reduce the mPAP and PVR, effects that are typically achieved in 3–6 months. A higher number of patients can be considered for heart transplantation after LVAD therapy with a possible benefit in post-transplant survival [231–236]. Although beneficial for the RV in the long term, LVADs can exacerbate or cause new-onset RVF because the decrease in LV end-diastolic volume will shift the interventricular septum to the left, increasing the RV end-diastolic volume, compromising its contractility [237]. Approximately 6–10 % of patients with a LVAD will require the implantation of a RVAD [238].
RVADs have been successfully used for the management of RVF in patients with RV infarct, after cardiac surgery, after LVAD implantation, and following heart transplantation [239–244]. RVADs will increase pressure but not sufficiently to overcome the increased RV afterload potentially injuring the lung [245]. BiVADs may be used in cases of bilateral ventricular failure as a bridge to transplantation [246].
Veno-arterial (V-A) ECMO has the ability to provide both cardiovascular and respiratory support as it drains deoxygenated blood from the venous circulation and returns oxygenated blood to the arterial circulation. V-A ECMO can be considered in patients with RVF secondary to increased afterload as a bridge to transplantation when medical interventions do not suffice. Although small studies suggest favorable outcomes with the use of ECMO, further research is needed before general recommendations can be made and its use should be reserved for centers with expertise [247].
Miscellaneous ICU situations
Given the improvement in survival in patients with PH produced by modern available therapies, an increase in other conditions that were not as common in this group of patients has occurred. The clinician must be familiar with the pathophysiologic differences of the PH patient to provide the best possible care.
Pregnant patient with PH
During pregnancy, significant physiologic changes occur including increased blood volume, increased CO, decreased SVR, and increased pulmonary blood flow [248]. During delivery, pain, anxiety, raised levels of catecholamine and uterine contractions produce an increase in CO. After delivery, the venous return increases significantly as a result of the involution of the uterus producing a sizeable increase in CO. Patients with normal PVR can readily tolerate these changes through vasodilation and recruitment of pulmonary vessels, whereas patients with PH cannot adapt making them prone to develop acute RVF [249]. Consequently, the mortality rate, albeit lower than in previous times, remains very high ranging from 17 to 28 %, with most complications occurring in the peripartum period [250, 251].
Vaginal delivery may be better tolerated by PH patients given the smaller shifts in blood volume and greater stability in hemodynamics, but cesarean section (CS) may become urgently necessary in cases of fetal distress or maternal deterioration. Recent literature reports a more frequent use of CS and a higher proportion of premature deliveries; this change in practice may be explained by closer surveillance and a lower threshold for intervention in cases of maternal deterioration or fetal distress [251]. A multidisciplinary team including the cardiac anesthesiologist, obstetrician, and neonatologist should be involved if a CS is the chosen route of delivery. The PH patient should undergo close monitoring during the peripartum period with particular attention to arterial oxygenation, cardiac rhythm, and hemodynamics. The use of invasive monitoring devices seems reasonable and should be considered on a case-by-case basis [252, 253]. Successful outcomes have been reported with the use of various pulmonary vasodilators during the peripartum period including epoprostenol, iNO, and combination of sildenafil and epoprostenol [254–259].
Liver disease and PH
PoPH is a complication of portal hypertension that occurs more commonly in patients with chronic liver disease. It is often asymptomatic and discovered during the perioperative period for liver transplantation when it poses the highest risk [260, 261]. The acute increase in CO at the time of reperfusion cannot be handled by the RV of the patient with PoPH leading to acute RVF [262]. The evaluation of risk, indications for advanced therapy, and contraindications to liver transplant are based on hemodynamic variables including mPAP and PVR. A mPAP > 50 is considered a contraindication for liver transplant, whereas a mPAP <35 is considered safe [263, 264]. Pulmonary vasodilators are used in the management of PoPH to improve hemodynamics. Several small studies report the use of prostacyclin analogs [265–267], ERAs [266, 268–270], and PDE5 inhibitors [268, 271, 272] in the management of patients with PoPH. In the perioperative period, combined use of iNO and epoprostenol has been reported with good outcomes [165, 166].
PH in biventricular failure
Left ventricular failure is among the most common causes of RVF [9]. The backflow caused by left heart disease increases LV end-diastolic pressure and RV afterload. Therefore, the treatment of patients with biventricular failure should focus on optimizing LV function through improvement in preload, contractility, and afterload. However, even after optimization of the left heart function, the transpulmonary gradient (mPAP–PCWP) may remain elevated, a phenomena known as “out of proportion” PH that is thought to be caused by an intrinsic abnormality in the pulmonary vasculature [273, 274]. The persistent elevation in PVR is especially important in patients being considered for cardiac transplant or LVAD placement because of an increased perioperative risk and decreased long-term survival after transplantation and the potential need for additional mechanical RV support [238, 275]. Sildenafil has been linked to improved exercise capacity and pulmonary hemodynamics in secondary PH patients with systolic heart failure, but not in patients with diastolic heart failure [276–278]. Prostacyclin analogs have also been used with positive hemodynamic changes [279, 280]. ERAs have not been associated with improved clinical outcomes and may increase the risk of decompensation [281–284]. Nevertheless, more evidence is needed before general recommendations can be made regarding the use of pulmonary vasodilators in this setting.
Sepsis
Sepsis poses a myriad of physiologic derangements including increased vascular permeability, vasodilation, hypovolemia, and decreased SVR that must be overcome by an increment in CO [4–6, 10]. Low SVR leads to decreased RV coronary perfusion, myocardial ischemia, and failure. In addition, there is often an increase in PVR causing decreased RV output. An increase in RV afterload or intrinsic myocardial depression may be the dominant cause of RV dysfunction in sepsis [4–6, 10]. Given the increased PVR, increasing cardiac output may prove very difficult in patients with PH and sepsis can trigger acute RVF. The management of the septic patient with PH must include early administration of antibiotics and hemodynamic optimization that should encompass the optimization of preload, enhancement of SVR, improvement of CO, and prevention of increase in PVR (as discussed above) [285]. Fluid resuscitation should not be liberal in this population and ideally must be guided by invasive hemodynamic monitoring devices. The use of pulmonary vasodilators in sepsis should be reserved to cases where further decrease in PVR is needed to improve cardiac output and systemic perfusion, keeping in mind that these agents produce systemic effects that could worsen the hemodynamic status.
Advance directives and resuscitation outcomes
The etiology of acute RVF largely influences the prognosis; patients with severe left heart disease or progressive PH in the setting of CTD have a worse prognosis compared to patients with acute PE [10, 286]. Despite the recent advancements in therapy that have improved the quality of life and survival in PH, it still remains a progressive disease that will ultimately have fatal outcomes. Therefore, it is important that the patient’s wishes pertaining to end-of-life care be discussed in a serene environment during the course of the disease. Grinnan et al. reported that the majority of patients with PAH died in the hospital and most of those deaths happened in the ICU [287]. Unfortunately, the ICU may not be the best setting to have this conversation for the first time when decompensation typically occurs rapidly and decisions may need to be made very quickly. Cardiopulmonary resuscitation (CPR) is attempted in 25 % of patients that ultimately die of progression of the disease. The survival of PH patients who arrested and had CPR is quite low (0–6 %) compared to the survival from other causes of cardiac arrest, a fact that is not surprising given that chronic disease is associated with poor outcomes after CPR [288, 289]. Moreover, almost every patient that survived had a correctable cause [290]. Recent evidence shows that palliative care services were infrequently utilized in the care of patients with PH [287, 291, 292].
The care of patients with progressive disease should include a discussion of goals of care, potentially limiting aggressive therapy, and referral to palliative care when appropriate for the management of symptoms and potentially improve quality of life.
Conclusions
PH and concomitant RVF present a diagnostic and therapeutic challenge in the ICU and have been associated with increased mortality. Prompt recognition is essential for the appropriate management in these patients. The use of biochemical markers and echocardiography may help in the diagnosis and have prognostic value, and invasive monitoring with PAC is likely necessary to monitor hemodynamics and therapeutic changes. Careful manipulation of RV preload is required as under- or overfilling of the RV will worsen its contractility. Pulmonary vasodilators have a profound effect on PVR and can be used to increase CO but co-administration of systemic vasopressors and restoration of cardiac output with the use of inotropes are usually required. Mechanical circulatory support is predominately being utilized as a bridge to transplant. Despite the advances in therapy that have improved the survival in patients with PH, realistic expectations should be discussed in the outpatient setting as patients who suffer cardiac arrest have very poor outcomes. Overall, more studies of RVF are required to improve overall outcomes of this disease.
References
Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB, National Heart L, Blood Institute Working Group on C, Molecular Mechanisms of Right Heart F (2006) Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114(17):1883–1891. doi:10.1161/CIRCULATIONAHA.106.632208
Kawut SM, Barr RG, Lima JA, Praestgaard A, Johnson WC, Chahal H, Ogunyankin KO, Bristow MR, Kizer JR, Tandri H, Bluemke DA (2012) Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi-Ethnic Study of Atherosclerosis (MESA)–right ventricle study. Circulation 126(14):1681–1688. doi:10.1161/CIRCULATIONAHA.112.095216
Hurford WE, Zapol WM (1988) The right ventricle and critical illness: a review of anatomy, physiology, and clinical evaluation of its function. Intensive Care Med 14(Suppl 2):448–457
Kumar A, Haery C, Parrillo JE (2000) Myocardial dysfunction in septic shock. Crit Care Clin 16(2):251–287
Mitsuo T, Shimazaki S, Matsuda H (1992) Right ventricular dysfunction in septic patients. Crit Care Med 20(5):630–634
Hoffman MJ, Greenfield LJ, Sugerman HJ, Tatum JL (1983) Unsuspected right ventricular dysfunction in shock and sepsis. Ann Surg 198(3):307–319
Sztrymf B, Souza R, Bertoletti L, Jais X, Sitbon O, Price LC, Simonneau G, Humbert M (2010) Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J 35(6):1286–1293. doi:10.1183/09031936.00070209
Haddad F, Peterson T, Fuh E, Kudelko KT, de Jesus Perez V, Skhiri M, Vagelos R, Schnittger I, Denault AY, Rosenthal DN, Doyle RL, Zamanian RT (2011) Characteristics and outcome after hospitalization for acute right heart failure in patients with pulmonary arterial hypertension. Circ Heart Fail 4(6):692–699. doi:10.1161/CIRCHEARTFAILURE.110.949933
Lahm T, McCaslin CA, Wozniak TC, Ghumman W, Fadl YY, Obeidat OS, Schwab K, Meldrum DR (2010) Medical and surgical treatment of acute right ventricular failure. J Am Coll Cardiol 56(18):1435–1446. doi:10.1016/j.jacc.2010.05.046
Kurzyna M, Zylkowska J, Fijalkowska A, Florczyk M, Wieteska M, Kacprzak A, Burakowski J, Szturmowicz M, Wawrzynska L, Torbicki A (2008) Characteristics and prognosis of patients with decompensated right ventricular failure during the course of pulmonary hypertension. Kardiol Pol 66(10):1033–1039 discussion 1040–1031
Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R (2013) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62(25 Suppl):D34–D41. doi:10.1016/j.jacc.2013.10.029
Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G (2006) Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 173(9):1023–1030. doi:10.1164/rccm.200510-1668OC
Escribano-Subias P, Blanco I, Lopez-Meseguer M, Lopez-Guarch CJ, Roman A, Morales P, Castillo-Palma MJ, Segovia J, Gomez-Sanchez MA, Barbera JA (2012) Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J 40(3):596–603. doi:10.1183/09031936.00101211
Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W (2013) Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 62(25 Suppl):D100–D108. doi:10.1016/j.jacc.2013.10.033
Seeger W, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs S, Martinez FJ, Semigran MJ, Simonneau G, Wells AU, Vachiery JL (2013) Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 62(25 Suppl):D109–D116. doi:10.1016/j.jacc.2013.10.036
Gustavo AH, Vijay NJ, David P, Margarita A, Rajesh K, Simon T, Carol S (2015) Demographic characteristics of group 3 pulmonary hypertension patients in United States. In: B108. Beyond Who Group I Pulmonary Hypertension. American Thoracic Society International Conference Abstracts. American Thoracic Society. doi:10.1164/ajrccm-conference.2015.191.1_MeetingAbstracts.A3835
Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, Prandoni P, Thromboembolic Pulmonary Hypertension Study G (2004) Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 350(22):2257–2264. doi:10.1056/NEJMoa032274
Piazza G, Goldhaber SZ (2011) Chronic thromboembolic pulmonary hypertension. N Engl J Med 364(4):351–360. doi:10.1056/NEJMra0910203
Naeije R (2005) Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2(1):20–22. doi:10.1513/pats.200407-037MS
Naeije R, Barbera JA (2001) Pulmonary hypertension associated with COPD. Crit Care 5(6):286–289
Weitzenblum E (2003) Chronic cor pulmonale. Heart 89(2):225–230
Ventetuolo CE, Klinger JR (2014) Management of acute right ventricular failure in the intensive care unit. Ann Am Thorac Soc 11(5):811–822. doi:10.1513/AnnalsATS.201312-446FR
Hoeper MM, Granton J (2011) Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med 184(10):1114–1124. doi:10.1164/rccm.201104-0662CI
Ducas J, Magder S, McGregor M (1983) Validity of the hepatojugular reflux as a clinical test for congestive heart failure. Am J Cardiol 52(10):1299–1303
Zamanian RT, Haddad F, Doyle RL, Weinacker AB (2007) Management strategies for patients with pulmonary hypertension in the intensive care unit. Crit Care Med 35(9):2037–2050
Bates B, Hoekelman RA, Thompson JB (1995) A guide to physical examination and history taking, 6th edn. Lippincott, Philadelphia
Forfia PR, Mathai SC, Fisher MR, Housten-Harris T, Hemnes AR, Champion HC, Girgis RE, Hassoun PM (2008) Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 177(12):1364–1369. doi:10.1164/rccm.200712-1876OC
Shah SJ, Thenappan T, Rich S, Tian L, Archer SL, Gomberg-Maitland M (2008) Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation 117(19):2475–2483. doi:10.1161/CIRCULATIONAHA.107.719500
Dorfmuller P, Perros F, Balabanian K, Humbert M (2003) Inflammation in pulmonary arterial hypertension. Eur Respir J 22(2):358–363
Quarck R, Nawrot T, Meyns B, Delcroix M (2009) C-reactive protein: a new predictor of adverse outcome in pulmonary arterial hypertension. J Am Coll Cardiol 53(14):1211–1218. doi:10.1016/j.jacc.2008.12.038
Leuchte HH, Holzapfel M, Baumgartner RA, Ding I, Neurohr C, Vogeser M, Kolbe T, Schwaiblmair M, Behr J (2004) Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol 43(5):764–770. doi:10.1016/j.jacc.2003.09.051
Reesink HJ, Tulevski II, Marcus JT, Boomsma F, Kloek JJ, Vonk Noordegraaf A, Bresser P (2007) Brain natriuretic peptide as noninvasive marker of the severity of right ventricular dysfunction in chronic thromboembolic pulmonary hypertension. Ann Thorac Surg 84(2):537–543. doi:10.1016/j.athoracsur.2007.04.006
Nagaya N, Nishikimi T, Okano Y, Uematsu M, Satoh T, Kyotani S, Kuribayashi S, Hamada S, Kakishita M, Nakanishi N, Takamiya M, Kunieda T, Matsuo H, Kangawa K (1998) Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol 31(1):202–208
Tung RH, Garcia C, Morss AM, Pino RM, Fifer MA, Thompson BT, Lewandrowski K, Lee-Lewandrowski E, Januzzi JL (2004) Utility of B-type natriuretic peptide for the evaluation of intensive care unit shock. Crit Care Med 32(8):1643–1647
Murninkas D, Alba AC, Delgado D, McDonald M, Billia F, Chan WS, Ross HJ (2014) Right ventricular function and prognosis in stable heart failure patients. J Cardiac Fail 20(5):343–349. doi:10.1016/j.cardfail.2014.01.018
Lega JC, Lacasse Y, Lakhal L, Provencher S (2009) Natriuretic peptides and troponins in pulmonary embolism: a meta-analysis. Thorax 64(10):869–875. doi:10.1136/thx.2008.110965
Choi HS, Kim KH, Yoon HJ, Hong YJ, Kim JH, Ahn Y, Jeong MH, Cho JG, Park JC, Kang JC (2012) Usefulness of cardiac biomarkers in the prediction of right ventricular dysfunction before echocardiography in acute pulmonary embolism. J Cardiol 60(6):508–513. doi:10.1016/j.jjcc.2012.07.006
Roy AK, McCullagh BN, Segurado R, McGorrian C, Keane E, Keaney J, Fitzgibbon MN, Mahon NG, Murray PT, Gaine SP (2014) Detection of high-sensitivity troponin in outpatients with stable pulmonary hypertension identifies a subgroup at higher risk of adverse outcomes. J Cardiac Fail 20(1):31–37. doi:10.1016/j.cardfail.2013.12.001
Schuuring MJ, van Riel AC, Vis JC, Duffels MG, van Straalen JP, Boekholdt SM, Tijssen JG, Mulder BJ, Bouma BJ (2013) High-sensitivity troponin T is associated with poor outcome in adults with pulmonary arterial hypertension due to congenital heart disease. Congenit Heart Dis 8(6):520–526. doi:10.1111/chd.12022
Filusch A, Giannitsis E, Katus HA, Meyer FJ (2010) High-sensitive troponin T: a novel biomarker for prognosis and disease severity in patients with pulmonary arterial hypertension. Clin Sci (Lond) 119(5):207–213. doi:10.1042/CS20100014
Becattini C, Vedovati MC, Agnelli G (2007) Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation 116(4):427–433. doi:10.1161/CIRCULATIONAHA.106.680421
Chronic Cor Pulmonale: Report of an Expert Committee (1963) Circulation 27(4):594–615. doi:10.1161/01.cir.27.4.594
Murphy ML, Thenabadu PN, de Soyza N, Doherty JE, Meade J, Baker BJ, Whittle JL (1984) Reevaluation of electrocardiographic criteria for left, right and combined cardiac ventricular hypertrophy. Am J Cardiol 53(8):1140–1147
Lehtonen J, Sutinen S, Ikaheimo M, Paakko P (1988) Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest 93(4):839–842
Bossone E, Paciocco G, Iarussi D, Agretto A, Iacono A, Gillespie BW, Rubenfire M (2002) The prognostic role of the ECG in primary pulmonary hypertension. Chest 121(2):513–518
Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, Gaine S (2004) Diagnosis and differential assessment of pulmonary arterial hypertension. Journal of the American College of Cardiology 43 (12 Suppl S):40S-47S. doi:10.1016/j.jacc.2004.02.032
Bourji KI, Hassoun PM (2015) Right ventricle dysfunction in pulmonary hypertension: mechanisms and modes of detection. Curr Opin Pulm Med. doi:10.1097/MCP.0000000000000192
Feigenbaum H, Armstrong WF, Ryan T, Feigenbaum H, Ovid Technologies Inc. (2005) Feigenbaum’s echocardiography
McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, American College of Cardiology Foundation Task Force on Expert Consensus D, American Heart A, American College of Chest P, American Thoracic Society I, Pulmonary Hypertension A (2009) ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. Journal of the American College of Cardiology 53 (17):1573–1619. doi:10.1016/j.jacc.2009.01.004
Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S (2011) Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 139(5):988–993. doi:10.1378/chest.10-1269
Janda S, Shahidi N, Gin K, Swiston J (2011) Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart 97(8):612–622. doi:10.1136/hrt.2010.212084
Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ (1985) Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol 6(4):750–756
Hinderliter AL, Willis PWt, Long WA, Clarke WR, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Koch G, Li S, Clayton LM, Jobsis MM, Crow JW, Group PPHS (2003) Frequency and severity of tricuspid regurgitation determined by Doppler echocardiography in primary pulmonary hypertension. Am J Cardiol 91(8):1033–1037, A1039
van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk-Noordegraaf A (2011) Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 58(24):2511–2519. doi:10.1016/j.jacc.2011.06.068
Anavekar NS, Gerson D, Skali H, Kwong RY, Yucel EK, Solomon SD (2007) Two-dimensional assessment of right ventricular function: an echocardiographic-MRI correlative study. Echocardiography 24(5):452–456. doi:10.1111/j.1540-8175.2007.00424.x
Schenk P, Globits S, Koller J, Brunner C, Artemiou O, Klepetko W, Burghuber OC (2000) Accuracy of echocardiographic right ventricular parameters in patients with different end-stage lung diseases prior to lung transplantation. J Heart Lung Transplant 19(2):145–154
Meluzin J, Spinarova L, Bakala J, Toman J, Krejci J, Hude P, Kara T, Soucek M (2001) Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion; a new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J 22(4):340–348. doi:10.1053/euhj.2000.2296
Ghio S, Pazzano AS, Klersy C, Scelsi L, Raineri C, Camporotondo R, D’Armini A, Visconti LO (2011) Clinical and prognostic relevance of echocardiographic evaluation of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol 107(4):628–632. doi:10.1016/j.amjcard.2010.10.027
Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM (2006) Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 174(9):1034–1041. doi:10.1164/rccm.200604-547OC
Lee CY, Chang SM, Hsiao SH, Tseng JC, Lin SK, Liu CP (2007) Right heart function and scleroderma: insights from tricuspid annular plane systolic excursion. Echocardiography 24(2):118–125. doi:10.1111/j.1540-8175.2007.00365.x
Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, Seward SB (1996) Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr 9(6):838–847
Yeo TC, Dujardin KS, Tei C, Mahoney DW, McGoon MD, Seward JB (1998) Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. Am J Cardiol 81(9):1157–1161
Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Schwartz T, Koch G, Clayton LM, Jobsis MM, Crow JW, Long W (2002) Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 39(7):1214–1219
Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, Ammash NM, McCully RB, Miller FA, Pellikka PA, Oh JK, Kane GC (2011) Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 139(6):1299–1309. doi:10.1378/chest.10-2015
Ernande L, Cottin V, Leroux PY, Girerd N, Huez S, Mulliez A, Bergerot C, Ovize M, Mornex JF, Cordier JF, Naeije R, Derumeaux G (2013) Right isovolumic contraction velocity predicts survival in pulmonary hypertension. J Am Soc Echocardiogr 26(3):297–306. doi:10.1016/j.echo.2012.11.011
Eysmann SB, Palevsky HI, Reichek N, Hackney K, Douglas PS (1989) Two-dimensional and Doppler-echocardiographic and cardiac catheterization correlates of survival in primary pulmonary hypertension. Circulation 80(2):353–360
Hinderliter AL, Willis PWt, Long W, Clarke WR, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Biosblanc B, Koch G, Li S, Clayton LM, Jobsis MM, Crow JW (1999) Frequency and prognostic significance of pericardial effusion in primary pulmonary hypertension. PPH Study Group. Primary pulmonary hypertension. Am J Cardiol 84 (4):481-484, A410
D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT et al (1991) Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115(5):343–349
McLaughlin VV, Shillington A, Rich S (2002) Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 106(12):1477–1482
Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Herve P, Rainisio M, Simonneau G (2002) Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 40(4):780–788
Sandoval J, Bauerle O, Palomar A, Gomez A, Martinez-Guerra ML, Beltran M, Guerrero ML (1994) Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation 89(4):1733–1744
Rich S, Kaufmann E, Levy PS (1992) The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 327(2):76–81. doi:10.1056/NEJM199207093270203
Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Herve P, Simonneau G (2005) Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 111(23):3105–3111. doi:10.1161/CIRCULATIONAHA.104.488486
Hoeper MM, Lee SH, Voswinckel R, Palazzini M, Jais X, Marinelli A, Barst RJ, Ghofrani HA, Jing ZC, Opitz C, Seyfarth HJ, Halank M, McLaughlin V, Oudiz RJ, Ewert R, Wilkens H, Kluge S, Bremer HC, Baroke E, Rubin LJ (2006) Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol 48(12):2546–2552. doi:10.1016/j.jacc.2006.07.061
Ranu H, Smith K, Nimako K, Sheth A, Madden BP (2010) A retrospective review to evaluate the safety of right heart catheterization via the internal jugular vein in the assessment of pulmonary hypertension. Clin Cardiol 33(5):303–306. doi:10.1002/clc.20770
Rhodes A, Cusack RJ, Newman PJ, Grounds RM, Bennett ED (2002) A randomised, controlled trial of the pulmonary artery catheter in critically ill patients. Intensive Care Med 28(3):256–264. doi:10.1007/s00134-002-1206-9
Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, Brampton W, Williams D, Young D, Rowan K (2005) Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet 366(9484):472–477. doi:10.1016/S0140-6736(05)67061-4
Schneider AJ, Teule GJ, Groeneveld AB, Nauta J, Heidendal GA, Thijs LG (1988) Biventricular performance during volume loading in patients with early septic shock, with emphasis on the right ventricle: a combined hemodynamic and radionuclide study. Am Heart J 116(1 Pt 1):103–112
Mercat A, Diehl JL, Meyer G, Teboul JL, Sors H (1999) Hemodynamic effects of fluid loading in acute massive pulmonary embolism. Crit Care Med 27(3):540–544
Belenkie I, Dani R, Smith ER, Tyberg JV (1989) Effects of volume loading during experimental acute pulmonary embolism. Circulation 80(1):178–188
Vlahakes GJ, Turley K, Hoffman JI (1981) The pathophysiology of failure in acute right ventricular hypertension: hemodynamic and biochemical correlations. Circulation 63(1):87–95
Santamore WP, Dell’Italia LJ (1998) Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis 40(4):289–308
Zwissler B (2000) [Acute right heart failure. Etiology–pathophysiology–diagnosis–therapy]. Der Anaesthesist 49(9):788–808
Leier CV, Heban PT, Huss P, Bush CA, Lewis RP (1978) Comparative systemic and regional hemodynamic effects of dopamine and dobutamine in patients with cardiomyopathic heart failure. Circulation 58(3 Pt 1):466–475
Bradford KK, Deb B, Pearl RG (2000) Combination therapy with inhaled nitric oxide and intravenous dobutamine during pulmonary hypertension in the rabbit. J Cardiovasc Pharmacol 36(2):146–151
Kerbaul F, Rondelet B, Motte S, Fesler P, Hubloue I, Ewalenko P, Naeije R, Brimioulle S (2004) Effects of norepinephrine and dobutamine on pressure load-induced right ventricular failure. Crit Care Med 32(4):1035–1040
Bryan TL, van Diepen S, Bhutani M, Shanks M, Welsh RC (1985) Stickland MK (2012) The effects of dobutamine and dopamine on intrapulmonary shunt and gas exchange in healthy humans. J Appl Physiol 113(4):541–548. doi:10.1152/japplphysiol.00404.2012
Vizza CD, Rocca GD, Roma AD, Iacoboni C, Pierconti F, Venuta F, Rendina E, Schmid G, Pietropaoli P, Fedele F (2001) Acute hemodynamic effects of inhaled nitric oxide, dobutamine and a combination of the two in patients with mild to moderate secondary pulmonary hypertension. Crit Care 5(6):355–361
Chen EP, Bittner HB, Davis RD Jr, Van Trigt P III (1997) Milrinone improves pulmonary hemodynamics and right ventricular function in chronic pulmonary hypertension. Ann Thorac Surg 63(3):814–821
Chen EP, Bittner HB, Davis RD, Van Trigt P (1998) Hemodynamic and inotropic effects of milrinone after heart transplantation in the setting of recipient pulmonary hypertension. J Heart Lung Transplant 17(7):669–678
Solina A, Papp D, Ginsberg S, Krause T, Grubb W, Scholz P, Pena LL, Cody R (2000) A comparison of inhaled nitric oxide and milrinone for the treatment of pulmonary hypertension in adult cardiac surgery patients. J Cardiothorac Vasc Anesth 14(1):12–17
Haglund NA, Burdorf A, Jones T, Shostrom V, Um J, Ryan T, Shillcutt S, Fischer P, Cox ZL, Raichlin E, Lowes BD, Dumitru I (2015) Inhaled milrinone after left ventricular assist device implantation. J Cardiac Fail. doi:10.1016/j.cardfail.2015.04.011
Lamarche Y, Malo O, Thorin E, Denault A, Carrier M, Roy J, Perrault LP (2005) Inhaled but not intravenous milrinone prevents pulmonary endothelial dysfunction after cardiopulmonary bypass. J Thorac Cardiovasc Surg 130(1):83–92. doi:10.1016/j.jtcvs.2004.09.011
Wang H, Gong M, Zhou B, Dai A (2009) Comparison of inhaled and intravenous milrinone in patients with pulmonary hypertension undergoing mitral valve surgery. Adv Ther 26(4):462–468. doi:10.1007/s12325-009-0019-4
Khazin V, Kaufman Y, Zabeeda D, Medalion B, Sasson L, Schachner A, Ezri T (2004) Milrinone and nitric oxide: combined effect on pulmonary artery pressures after cardiopulmonary bypass in children. J Cardiothorac Vasc Anesth 18(2):156–159
Vildbrad MD, Andersen A, Holmboe S, Ringgaard S, Nielsen JM, Nielsen-Kudsk JE (2014) Acute effects of levosimendan in experimental models of right ventricular hypertrophy and failure. Pulm Circ 4(3):511–519. doi:10.1086/677366
Kerbaul F, Gariboldi V, Giorgi R, Mekkaoui C, Guieu R, Fesler P, Gouin F, Brimioulle S, Collart F (2007) Effects of levosimendan on acute pulmonary embolism-induced right ventricular failure. Crit Care Med 35(8):1948–1954. doi:10.1097/01.CCM.0000275266.33910.8D
Morelli A, Teboul JL, Maggiore SM, Vieillard-Baron A, Rocco M, Conti G, De Gaetano A, Picchini U, Orecchioni A, Carbone I, Tritapepe L, Pietropaoli P, Westphal M (2006) Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit Care Med 34(9):2287–2293. doi:10.1097/01.CCM.0000230244.17174.4F
Kerbaul F, Rondelet B, Demester JP, Fesler P, Huez S, Naeije R, Brimioulle S (2006) Effects of levosimendan versus dobutamine on pressure load-induced right ventricular failure. Crit Care Med 34(11):2814–2819. doi:10.1097/01.CCM.0000242157.19347.50
Kaheinen P, Pollesello P, Levijoki J, Haikala H (2004) Effects of levosimendan and milrinone on oxygen consumption in isolated guinea-pig heart. J Cardiovasc Pharmacol 43(4):555–561
Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L (2002) Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 360(9328):196–202
Moiseyev VS, Poder P, Andrejevs N, Ruda MY, Golikov AP, Lazebnik LB, Kobalava ZD, Lehtonen LA, Laine T, Nieminen MS, Lie KI (2002) Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J 23(18):1422–1432
Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Poder P, Kivikko M (2007) Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 297(17):1883–1891. doi:10.1001/jama.297.17.1883
Lowensohn HS, Khouri EM, Gregg DE, Pyle RL, Patterson RE (1976) Phasic right coronary artery blood flow in conscious dogs with normal and elevated right ventricular pressures. Circ Res 39(6):760–766
Bergofsky EH (1980) Humoral control of the pulmonary circulation. Annu Rev Physiol 42:221–233. doi:10.1146/annurev.ph.42.030180.001253
Kwak YL, Lee CS, Park YH, Hong YW (2002) The effect of phenylephrine and norepinephrine in patients with chronic pulmonary hypertension*. Anaesthesia 57(1):9–14
Schreuder WO, Schneider AJ, Groeneveld AB, Thijs LG (1989) Effect of dopamine versus norepinephrine on hemodynamics in septic shock. Emphasis on right ventricular performance. Chest 95(6):1282–1288
Hirsch LJ, Rooney MW, Wat SS, Kleinmann B, Mathru M (1991) Norepinephrine and phenylephrine effects on right ventricular function in experimental canine pulmonary embolism. Chest 100(3):796–801
Bouissou A, Rakza T, Klosowski S, Tourneux P, Vanderborght M, Storme L (2008) Hypotension in preterm infants with significant patent ductus arteriosus: effects of dopamine. J Pediatr 153(6):790–794. doi:10.1016/j.jpeds.2008.06.014
Liet JM, Boscher C, Gras-Leguen C, Gournay V, Debillon T, Roze JC (2002) Dopamine effects on pulmonary artery pressure in hypotensive preterm infants with patent ductus arteriosus. J Pediatr 140(3):373–375. doi:10.1067/mpd.2002.123100
Lejeune P, Leeman M, Deloof T, Naeije R (1987) Pulmonary hemodynamic response to dopamine and dobutamine in hyperoxic and in hypoxic dogs. Anesthesiology 66(1):49–54
Lejeune P, Naeije R, Leeman M, Melot C, Deloof T, Delcroix M (1987) Effects of dopamine and dobutamine on hyperoxic and hypoxic pulmonary vascular tone in dogs. Am Rev Respir Dis 136(1):29–35. doi:10.1164/ajrccm/136.1.29
De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL, Investigators SI (2010) Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 362(9):779–789. doi:10.1056/NEJMoa0907118
Holloway EL, Polumbo RA, Harrison DC (1975) Acute circulatory effects of dopamine in patients with pulmonary hypertension. Br Heart J 37(5):482–485
Barrington KJ, Finer NN, Chan WK (1995) A blind, randomized comparison of the circulatory effects of dopamine and epinephrine infusions in the newborn piglet during normoxia and hypoxia. Crit Care Med 23(4):740–748
Le Tulzo Y, Seguin P, Gacouin A, Camus C, Suprin E, Jouannic I, Thomas R (1997) Effects of epinephrine on right ventricular function in patients with severe septic shock and right ventricular failure: a preliminary descriptive study. Intensive Care Med 23(6):664–670
Rich S, Gubin S, Hart K (1990) The effects of phenylephrine on right ventricular performance in patients with pulmonary hypertension. Chest 98(5):1102–1106
Walker BR, Haynes J Jr, Wang HL, Voelkel NF (1989) Vasopressin-induced pulmonary vasodilation in rats. Am J Physiol 257(2 Pt 2):H415–H422
Currigan DA, Hughes RJ, Wright CE, Angus JA, Soeding PF (2014) Vasoconstrictor responses to vasopressor agents in human pulmonary and radial arteries: an in vitro study. Anesthesiology 121(5):930–936. doi:10.1097/ALN.0000000000000430
Nagamine Y, Hara M (2012) Intravenous arginine vasopressin for two pediatric cases of pulmonary hypertension after congenital heart surgery. Masui 61(10):1112–1116
Tayama E, Ueda T, Shojima T, Akasu K, Oda T, Fukunaga S, Akashi H, Aoyagi S (2007) Arginine vasopressin is an ideal drug after cardiac surgery for the management of low systemic vascular resistant hypotension concomitant with pulmonary hypertension. Interact CardioVasc Thorac Surg 6(6):715–719. doi:10.1510/icvts.2007.159624
Jeon Y, Ryu JH, Lim YJ, Kim CS, Bahk JH, Yoon SZ, Choi JY (2006) Comparative hemodynamic effects of vasopressin and norepinephrine after milrinone-induced hypotension in off-pump coronary artery bypass surgical patients. Eur J Cardiothorac Surg 29(6):952–956. doi:10.1016/j.ejcts.2006.02.032
Sarkar J, Golden PJ, Kajiura LN, Murata LA, Uyehara CF (2015) Vasopressin decreases pulmonary-to-systemic vascular resistance ratio in a porcine model of severe hemorrhagic shock. Shock 43(5):475–482. doi:10.1097/SHK.0000000000000325
Mohamed A, Nasef N, Shah V, McNamara PJ (2014) Vasopressin as a rescue therapy for refractory pulmonary hypertension in neonates: case series. Pediatr Crit Care Med 15(2):148–154. doi:10.1097/PCC.0b013e31829f5fce
Price LC, Forrest P, Sodhi V, Adamson DL, Nelson-Piercy C, Lucey M, Howard LS (2007) Use of vasopressin after Caesarean section in idiopathic pulmonary arterial hypertension. Br J Anaesth 99(4):552–555. doi:10.1093/bja/aem180
Braun EB, Palin CA, Hogue CW (2004) Vasopressin during spinal anesthesia in a patient with primary pulmonary hypertension treated with intravenous epoprostenol. Anesth Analg 99(1):36–37
Leather HA, Segers P, Berends N, Vandermeersch E, Wouters PF (2002) Effects of vasopressin on right ventricular function in an experimental model of acute pulmonary hypertension. Crit Care Med 30(11):2548–2552. doi:10.1097/01.CCM.0000034696.32358.54
Mols P, Hallemans R, Van Kuyk M, Melot C, Lejeune P, Ham H, Vertongen F, Naeije R (1984) Hemodynamic effects of vasopressin, alone and in combination with nitroprusside, in patients with liver cirrhosis and portal hypertension. Ann Surg 199(2):176–181
Wilson MF, Brackett DJ, Archer LT, Hinshaw LB (1980) Mechanisms of impaired cardiac function by vasopressin. Ann Surg 191(4):494–500
Boyle WA 3rd, Segel LD (1986) Direct cardiac effects of vasopressin and their reversal by a vascular antagonist. Am J Physiol 251(4 Pt 2):H734–H741
Ichinose F, Roberts JD Jr, Zapol WM (2004) Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation 109(25):3106–3111. doi:10.1161/01.CIR.0000134595.80170.62
Cooper CE (1999) Nitric oxide and iron proteins. Biochim Biophys Acta 1411(2–3):290–309
Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J (1991) Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet 338(8776):1173–1174
Frostell CG, Blomqvist H, Hedenstierna G, Lundberg J, Zapol WM (1993) Inhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilation. Anesthesiology 78(3):427–435
Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM (1993) Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med 328(6):399–405. doi:10.1056/NEJM199302113280605
Qiu HB, Chen DC, Pan JQ, Liu DW, Ma S (1999) Inhibitory effects of nitric oxide and interleukin-10 on production of tumor necrosis factor alpha, interleukin-1 beta, and interleukin-6 in mouse alveolar macrophages. Zhongguo Yao Li Xue Bao 20(3):271–275
Meldrum DR, Shames BD, Meng X, Fullerton DA, McIntyre RC Jr, Grover FL, Harken AH (1998) Nitric oxide downregulates lung macrophage inflammatory cytokine production. Ann Thorac Surg 66(2):313–317
Hsu CW, Lee DL, Lin SL, Sun SF, Chang HW (2008) The initial response to inhaled nitric oxide treatment for intensive care unit patients with acute respiratory distress syndrome. Respiration 75(3):288–295. doi:10.1159/000101478
Ardehali A, Hughes K, Sadeghi A, Esmailian F, Marelli D, Moriguchi J, Hamilton MA, Kobashigawa J, Laks H (2001) Inhaled nitric oxide for pulmonary hypertension after heart transplantation. Transplantation 72(4):638–641
Fernandez-Perez ER, Keegan MT, Harrison BA (2006) Inhaled nitric oxide for acute right-ventricular dysfunction after extrapleural pneumonectomy. Respir Care 51(10):1172–1176
Healy DG, Veerasingam D, McHale J, Luke D (2006) Successful perioperative utilisation of inhaled nitric oxide in mitral valve surgery. J Cardiovasc Surg (Torino) 47(2):217–220
Meaudre E, Goutorbe P, Boret H, Kaiser E, Salinier L, Cantais E, Palmier B (2005) Nitric oxide inhalation is useful in the management of right ventricular failure caused by myocardial contusion. Acta Anaesthesiol Scand 49(3):415–417. doi:10.1111/j.1399-6576.2005.00644.x
Inglessis I, Shin JT, Lepore JJ, Palacios IF, Zapol WM, Bloch KD, Semigran MJ (2004) Hemodynamic effects of inhaled nitric oxide in right ventricular myocardial infarction and cardiogenic shock. J Am Coll Cardiol 44(4):793–798. doi:10.1016/j.jacc.2004.05.047
Fujita Y, Nishida O, Sobue K, Ito H, Kusama N, Inagaki M, Katsuya H (2002) Nitric oxide inhalation is useful in the management of right ventricular failure caused by myocardial infarction. Crit Care Med 30(6):1379–1381
Paniagua MJ, Crespo-Leiro MG, Rodriguez JA, Fojon S, Pastor J, Castro MJ, Hermida LF, Cuenca JJ, Juffe-Stein A, Castro-Beiras A (1999) Usefulness of nitric oxide inhalation for management of right ventricular failure after heart transplantation in patients with pretransplant pulmonary hypertension. Transplant Proc 31(6):2505–2506
Mosquera I, Crespo-Leiro MG, Tabuyo T, Paniagua MJ, Fuente L, Bouzas B, Fojon S, Pastor J, Juffe-Stein A, Castro-Beiras A (2002) Pulmonary hypertension and right ventricular failure after heart transplantation: usefulness of nitric oxide. Transplant Proc 34(1):166–167
Maxey TS, Smith CD, Kern JA, Tribble CG, Jones DR, Kron IL, Crosby IK (2002) Beneficial effects of inhaled nitric oxide in adult cardiac surgical patients. Ann Thorac Surg 73(2):529–532 discussion 532–523
Bhorade S, Christenson J, O’Connor M, Lavoie A, Pohlman A, Hall JB (1999) Response to inhaled nitric oxide in patients with acute right heart syndrome. Am J Respir Crit Care Med 159(2):571–579. doi:10.1164/ajrccm.159.2.9804127
Wagner F, Dandel M, Gunther G, Loebe M, Schulze-Neick I, Laucke U, Kuhly R, Weng Y, Hetzer R (1997) Nitric oxide inhalation in the treatment of right ventricular dysfunction following left ventricular assist device implantation. Circulation 96(9 Suppl):II-291–II-296
George SJ, Boscoe MJ (1997) Inhaled nitric oxide for right ventricular dysfunction following cardiac transplantation. Br J Clin Pract 51(1):53–55
Summerfield DT, Desai H, Levitov A, Grooms DA, Marik PE (2012) Inhaled nitric oxide as salvage therapy in massive pulmonary embolism: a case series. Respir Care 57(3):444–448. doi:10.4187/respcare.01373
Capellier G, Jacques T, Balvay P, Blasco G, Belle E, Barale F (1997) Inhaled nitric oxide in patients with pulmonary embolism. Intensive Care Med 23(10):1089–1092
Szold O, Khoury W, Biderman P, Klausner JM, Halpern P, Weinbroum AA (2006) Inhaled nitric oxide improves pulmonary functions following massive pulmonary embolism: a report of four patients and review of the literature. Lung 184(1):1–5. doi:10.1007/s00408-005-2550-7
Trummer G, Berchtold-Herz M, Martin J, Beyersdorf F (2002) Successful treatment of pulmonary hypertension with inhaled nitric oxide after pulmonary embolectomy. Ann Thorac Surg 73(4):1299–1301
Schenk P, Mittermayer C, Ratheiser K (1999) Inhaled nitric oxide in a patient with severe pulmonary embolism. Ann Emerg Med 33(6):710–714
Syed AU, Jelly AE, Algebaly AA, Altoonisi MM, Shatoory AE (2013) Methemoglobinemia due to nitric oxide therapy in a child after cardiac surgery. Asian Cardiovasc Thorac Ann 21(3):345–347. doi:10.1177/0218492312454276
Taylor MB, Christian KG, Patel N, Churchwell KB (2001) Methemoglobinemia: toxicity of inhaled nitric oxide therapy. Pediatr Crit Care Med 2(1):99–101
Griffiths MJ, Evans TW (2005) Inhaled nitric oxide therapy in adults. N Engl J Med 353(25):2683–2695. doi:10.1056/NEJMra051884
Christenson J, Lavoie A, O’Connor M, Bhorade S, Pohlman A, Hall JB (2000) The incidence and pathogenesis of cardiopulmonary deterioration after abrupt withdrawal of inhaled nitric oxide. Am J Respir Crit Care Med 161(5):1443–1449. doi:10.1164/ajrccm.161.5.9806138
Lavoie A, Hall JB, Olson DM, Wylam ME (1996) Life-threatening effects of discontinuing inhaled nitric oxide in severe respiratory failure. Am J Respir Crit Care Med 153(6 Pt 1):1985–1987. doi:10.1164/ajrccm.153.6.8665066
Atz AM, Adatia I, Wessel DL (1996) Rebound pulmonary hypertension after inhalation of nitric oxide. Ann Thorac Surg 62(6):1759–1764
Adhikari NK, Dellinger RP, Lundin S, Payen D, Vallet B, Gerlach H, Park KJ, Mehta S, Slutsky AS, Friedrich JO (2014) Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med 42(2):404–412. doi:10.1097/CCM.0b013e3182a27909
Afshari A, Brok J, Moller AM, Wetterslev J (2010) Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database Syst Rev (7):CD002787. doi:10.1002/14651858.CD002787.pub2
Westphal K, Martens S, Strouhal U, Matheis G, Hommel K, Kessler P (1998) Nitric oxide inhalation in acute pulmonary hypertension after cardiac surgery reduces oxygen concentration and improves mechanical ventilation but not mortality. Thorac Cardiovasc Surg 46(2):70–73. doi:10.1055/s-2007-1010192
Vater Y, Martay K, Dembo G, Bowdle TA, Weinbroum AA (2006) Intraoperative epoprostenol and nitric oxide for severe pulmonary hypertension during orthotopic liver transplantation: a case report and review of the literature. Med Sci Monit 12(12):CS115–CS118
Ramsay MA, Spikes C, East CA, Lynch K, Hein HA, Ramsay KJ, Klintmalm GB (1999) The perioperative management of portopulmonary hypertension with nitric oxide and epoprostenol. Anesthesiology 90(1):299–301
Antoniou T, Koletsis EN, Prokakis C, Rellia P, Thanopoulos A, Theodoraki K, Zarkalis D, Sfyrakis P (2013) Hemodynamic effects of combination therapy with inhaled nitric oxide and iloprost in patients with pulmonary hypertension and right ventricular dysfunction after high-risk cardiac surgery. J Cardiothorac Vasc Anesth 27(3):459–466. doi:10.1053/j.jvca.2012.07.020
Antoniou T, Prokakis C, Athanasopoulos G, Thanopoulos A, Rellia P, Zarkalis D, Kogerakis N, Koletsis EN, Bairaktaris A (2012) Inhaled nitric oxide plus iloprost in the setting of post-left assist device right heart dysfunction. Ann Thorac Surg 94(3):792–798. doi:10.1016/j.athoracsur.2012.04.046
Flondor M, Merkel M, Hofstetter C, Irlbeck M, Frey L, Zwissler B (2006) The effect of inhaled nitric oxide and inhaled iloprost on hypoxaemia in a patient with pulmonary hypertension after pulmonary thrombarterectomy. Anaesthesia 61(12):1200–1203. doi:10.1111/j.1365-2044.2006.04861.x
Wharton J, Strange JW, Moller GM, Growcott EJ, Ren X, Franklyn AP, Phillips SC, Wilkins MR (2005) Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med 172(1):105–113. doi:10.1164/rccm.200411-1587OC
Preston IR, Klinger JR, Houtches J, Nelson D, Farber HW, Hill NS (2005) Acute and chronic effects of sildenafil in patients with pulmonary arterial hypertension. Respir Med 99(12):1501–1510. doi:10.1016/j.rmed.2005.03.026
Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S (2002) Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation 105(20):2398–2403
Lepore JJ, Maroo A, Pereira NL, Ginns LC, Dec GW, Zapol WM, Bloch KD, Semigran MJ (2002) Effect of sildenafil on the acute pulmonary vasodilator response to inhaled nitric oxide in adults with primary pulmonary hypertension. Am J Cardiol 90(6):677–680
Madden BP, Sheth A, Ho TB, Park JE, Kanagasabay RR (2004) Potential role for sildenafil in the management of perioperative pulmonary hypertension and right ventricular dysfunction after cardiac surgery. Br J Anaesth 93(1):155–156. doi:10.1093/bja/aeh571
Tedford RJ, Hemnes AR, Russell SD, Wittstein IS, Mahmud M, Zaiman AL, Mathai SC, Thiemann DR, Hassoun PM, Girgis RE, Orens JB, Shah AS, Yuh D, Conte JV, Champion HC (2008) PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support. Circ Heart Fail 1(4):213–219. doi:10.1161/CIRCHEARTFAILURE.108.796789
Singh RK, Richmond ME, Zuckerman WA, Lee TM, Giblin TB, Rodriguez R, Chen JM, Addonizio LJ (2014) The use of oral sildenafil for management of right ventricular dysfunction after pediatric heart transplantation. Am J Transplant 14(2):453–458. doi:10.1111/ajt.12552
De Santo LS, Mastroianni C, Romano G, Amarelli C, Marra C, Maiello C, Galdieri N, Della Corte A, Cotrufo M, Caianiello G (2008) Role of sildenafil in acute posttransplant right ventricular dysfunction: successful experience in 13 consecutive patients. Transplant Proc 40(6):2015–2018. doi:10.1016/j.transproceed.2008.05.055
Boffini M, Sansone F, Ceresa F, Ribezzo M, Patane F, Comoglio C, Rinaldi M (2009) Role of oral sildenafil in the treatment of right ventricular dysfunction after heart transplantation. Transplant Proc 41(4):1353–1356. doi:10.1016/j.transproceed.2009.03.042
Trachte AL, Lobato EB, Urdaneta F, Hess PJ, Klodell CT, Martin TD, Staples ED, Beaver TM (2005) Oral sildenafil reduces pulmonary hypertension after cardiac surgery. Ann Thorac Surg 79(1):194–197. doi:10.1016/j.athoracsur.2004.06.086 discussion 194–197
Lee JE, Hillier SC, Knoderer CA (2008) Use of sildenafil to facilitate weaning from inhaled nitric oxide in children with pulmonary hypertension following surgery for congenital heart disease. J Intensive Care Med 23(5):329–334. doi:10.1177/0885066608321389
Namachivayam P, Theilen U, Butt WW, Cooper SM, Penny DJ, Shekerdemian LS (2006) Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. Am J Respir Crit Care Med 174(9):1042–1047. doi:10.1164/rccm.200605-694OC
Ruan CH, Dixon RA, Willerson JT, Ruan KH (2010) Prostacyclin therapy for pulmonary arterial hypertension. Texas Heart Institute journal/from the Texas Heart Institute of St Luke’s Episcopal Hospital, Texas Children’s Hospital 37(4):391–399
Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, Koerner SK, Langleben D, Keller CA, Murali S, Uretsky BF, Clayton LM, Jobsis MM, Blackburn SD, Shortino D, Crow JW (1996) A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 334(5):296–301. doi:10.1056/NEJM199602013340504
McLaughlin VV, Gaine SP, Barst RJ, Oudiz RJ, Bourge RC, Frost A, Robbins IM, Tapson VF, McGoon MD, Badesch DB, Sigman J, Roscigno R, Blackburn SD, Arneson C, Rubin LJ, Rich S (2003) Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharmacol 41(2):293–299
Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, Winkler J, Sitbon O, Popov W, Ghofrani HA, Manes A, Kiely DG, Ewert R, Meyer A, Corris PA, Delcroix M, Gomez-Sanchez M, Siedentop H, Seeger W (2002) Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 347(5):322–329. doi:10.1056/NEJMoa020204
Muzaffar S, Shukla N, Angelini GD, Jeremy JY (2004) Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right-heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. J Thorac Cardiovasc Surg 128(6):949–950. doi:10.1016/j.jtcvs.2004.05.035
De Wet CJ, Affleck DG, Jacobsohn E, Avidan MS, Tymkew H, Hill LL, Zanaboni PB, Moazami N, Smith JR (2004) Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. J Thorac Cardiovasc Surg 127(4):1058–1067. doi:10.1016/j.jtcvs.2003.11.035
Schroeder RA, Wood GL, Plotkin JS, Kuo PC (2000) Intraoperative use of inhaled PGI(2) for acute pulmonary hypertension and right ventricular failure. Anesth Analg 91(2):291–295
Fattouch K, Sbraga F, Bianco G, Speziale G, Gucciardo M, Sampognaro R, Ruvolo G (2005) Inhaled prostacyclin, nitric oxide, and nitroprusside in pulmonary hypertension after mitral valve replacement. J Card Surg 20(2):171–176. doi:10.1111/j.0886-0440.2005.200383w.x
Rex S, Schaelte G, Metzelder S, Flier S, de Waal EE, Autschbach R, Rossaint R, Buhre W (2008) Inhaled iloprost to control pulmonary artery hypertension in patients undergoing mitral valve surgery: a prospective, randomized-controlled trial. Acta Anaesthesiol Scand 52(1):65–72. doi:10.1111/j.1399-6576.2007.01476.x
Haraldsson A, Kieler-Jensen N, Ricksten SE (1996) Inhaled prostacyclin for treatment of pulmonary hypertension after cardiac surgery or heart transplantation: a pharmacodynamic study. J Cardiothorac Vasc Anesth 10(7):864–868
Theodoraki K, Rellia P, Thanopoulos A, Tsourelis L, Zarkalis D, Sfyrakis P, Antoniou T (2002) Inhaled iloprost controls pulmonary hypertension after cardiopulmonary bypass. Cana J Anaesth 49(9):963–967. doi:10.1007/BF03016884
Elliott CG, Palevsky HI (2004) Treatment with epoprostenol of pulmonary arterial hypertension following mitral valve replacement for mitral stenosis. Thorax 59(6):536–537
Khan TA, Schnickel G, Ross D, Bastani S, Laks H, Esmailian F, Marelli D, Beygui R, Shemin R, Watson L, Vartapetian I, Ardehali A (2009) A prospective, randomized, crossover pilot study of inhaled nitric oxide versus inhaled prostacyclin in heart transplant and lung transplant recipients. J Thorac Cardiovasc Surg 138(6):1417–1424. doi:10.1016/j.jtcvs.2009.04.063
Webb SA, Stott S, van Heerden PV (1996) The use of inhaled aerosolized prostacyclin (IAP) in the treatment of pulmonary hypertension secondary to pulmonary embolism. Intensive Care Med 22(4):353–355
Siobal MS, Hess DR (2010) Are inhaled vasodilators useful in acute lung injury and acute respiratory distress syndrome? Respir Care 55(2):144–157 discussion 157–161
Afshari A, Brok J, Moller AM, Wetterslev J (2010) Aerosolized prostacyclin for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Cochrane Database Syst Rev (8):CD007733. doi:10.1002/14651858.CD007733.pub2
Dzierba AL, Abel EE, Buckley MS, Lat I (2014) A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy 34(3):279–290
Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ (2001) Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 358(9288):1119–1123. doi:10.1016/S0140-6736(01)06250-X
Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G (2002) Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 346(12):896–903. doi:10.1056/NEJMoa012212
Ghofrani HA, Galie N, Grimminger F, Grunig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ (2013) Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 369(4):330–340. doi:10.1056/NEJMoa1209655
Enson Y, Giuntini C, Lewis ML, Morris TQ, Ferrer MI, Harvey RM (1964) The influence of hydrogen ion concentration and hypoxia on the pulmonary circulation. J Clin Invest 43:1146–1162. doi:10.1172/JCI104999
Rudolph AM, Yuan S (1966) Response of the pulmonary vasculature to hypoxia and H + ion concentration changes. J Clin Invest 45(3):399–411. doi:10.1172/JCI105355
Rose CE Jr, Van Benthuysen K, Jackson JT, Tucker CE, Kaiser DL, Grover RF, Weil JV (1983) Right ventricular performance during increased afterload impaired by hypercapnic acidosis in conscious dogs. Circ Res 52(1):76–84
Viitanen A, Salmenpera M, Heinonen J (1990) Right ventricular response to hypercarbia after cardiac surgery. Anesthesiology 73(3):393–400
Cherpanath TG, Lagrand WK, Schultz MJ, Groeneveld AB (2013) Cardiopulmonary interactions during mechanical ventilation in critically ill patients. Neth Heart J 21(4):166–172. doi:10.1007/s12471-013-0383-1
Jardin F, Delorme G, Hardy A, Auvert B, Beauchet A, Bourdarias JP (1990) Reevaluation of hemodynamic consequences of positive pressure ventilation: emphasis on cyclic right ventricular afterloading by mechanical lung inflation. Anesthesiology 72(6):966–970
Vieillard-Baron A, Loubieres Y, Schmitt JM, Page B, Dubourg O (1985) Jardin F (1999) Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol 87(5):1644–1650
Tidal L (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342(18):1301–1308. doi:10.1056/NEJM200005043421801
Jardin F, Vieillard-Baron A (2007) Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med 33(3):444–447. doi:10.1007/s00134-007-0552-z
Repesse X, Charron C, Vieillard-Baron A (2012) Right ventricular failure in acute lung injury and acute respiratory distress syndrome. Minerva Anestesiol 78(8):941–948
Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, Beauchet A, Jardin F (2001) Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med 29(8):1551–1555
Bull TM, Clark B, McFann K, Moss M (2010) Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med 182(9):1123–1128. doi:10.1164/rccm.201002-0250OC
Boissier F, Katsahian S, Razazi K, Thille AW, Roche-Campo F, Leon R, Vivier E, Brochard L, Vieillard-Baron A, Brun-Buisson C, Mekontso Dessap A (2013) Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med 39(10):1725–1733. doi:10.1007/s00134-013-2941-9
Vieillard-Baron A, Jardin F (2003) Why protect the right ventricle in patients with acute respiratory distress syndrome? Curr Opin Crit Care 9(1):15–21
Vieillard-Baron A, Charron C, Caille V, Belliard G, Page B, Jardin F (2007) Prone positioning unloads the right ventricle in severe ARDS. Chest 132(5):1440–1446. doi:10.1378/chest.07-1013
Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L (2013) Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368(23):2159–2168. doi:10.1056/NEJMoa1214103
David M, von Bardeleben RS, Weiler N, Markstaller K, Scholz A, Karmrodt J, Eberle B (2004) Cardiac function and haemodynamics during transition to high-frequency oscillatory ventilation. Eur J Anaesthesiol 21(12):944–952
Guervilly C, Forel JM, Hraiech S, Demory D, Allardet-Servent J, Adda M, Barreau-Baumstark K, Castanier M, Papazian L, Roch A (2012) Right ventricular function during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med 40(5):1539–1545. doi:10.1097/CCM.0b013e3182451b4a
Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, Rowan K, Cuthbertson BH (2013) High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 368(9):806–813. doi:10.1056/NEJMoa1215716
Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, Granton JT, Arabi YM, Arroliga AC, Stewart TE, Slutsky AS, Meade MO (2013) High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 368(9):795–805. doi:10.1056/NEJMoa1215554
Tongers J, Schwerdtfeger B, Klein G, Kempf T, Schaefer A, Knapp JM, Niehaus M, Korte T, Hoeper MM (2007) Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am Heart J 153(1):127–132. doi:10.1016/j.ahj.2006.09.008
Goldstein JA, Harada A, Yagi Y, Barzilai B, Cox JL (1990) Hemodynamic importance of systolic ventricular interaction, augmented right atrial contractility and atrioventricular synchrony in acute right ventricular dysfunction. J Am Coll Cardiol 16(1):181–189
Topol EJ, Goldschlager N, Ports TA, Dicarlo LA Jr, Schiller NB, Botvinick EH, Chatterjee K (1982) Hemodynamic benefit of atrial pacing in right ventricular myocardial infarction. Ann Intern Med 96(5):594–597
Love JC, Haffajee CI, Gore JM, Alpert JS (1984) Reversibility of hypotension and shock by atrial or atrioventricular sequential pacing in patients with right ventricular infarction. Am Heart J 108(1):5–13
Abraham KA, Brown MA, Norris RM (1985) Right ventricular infarction, bradyarrhythmias, and cardiogenic shock: importance of atrial or atrioventricular sequential pacing. Aust N Z J Med 15(1):52–54
Burks JM, Calder JR Jr, Roland DL (1979) Sinus arrest in diaphragmatic myocardial infarction: treatment of power failure with atrial pacing. PACE 2(6):553–559
Nader DA, Ceretto WJ, Vieweg WV (1981) Atrial pacing in the management of right ventricular infarction. South Med J 74(3):362–363
Janousek J, Tomek V, Chaloupecky VA, Reich O, Gebauer RA, Kautzner J, Hucin B (2004) Cardiac resynchronization therapy: a novel adjunct to the treatment and prevention of systemic right ventricular failure. J Am Coll Cardiol 44(9):1927–1931. doi:10.1016/j.jacc.2004.08.044
Dubin AM, Feinstein JA, Reddy VM, Hanley FL, Van Hare GF, Rosenthal DN (2003) Electrical resynchronization: a novel therapy for the failing right ventricle. Circulation 107(18):2287–2289. doi:10.1161/01.CIR.0000070930.33499.9F
Zimpfer D, Zrunek P, Sandner S, Schima H, Grimm M, Zuckermann A, Wolner E, Wieselthaler G (2007) Post-transplant survival after lowering fixed pulmonary hypertension using left ventricular assist devices. Eur J Cardiothorac Surg 31(4):698–702. doi:10.1016/j.ejcts.2006.12.036
Liden H, Haraldsson A, Ricksten SE, Kjellman U, Wiklund L (2009) Does pretransplant left ventricular assist device therapy improve results after heart transplantation in patients with elevated pulmonary vascular resistance? Eur J Cardiothorac Surg 35 (6):1029-1034; discussion 1034-1025. doi:10.1016/j.ejcts.2008.12.024
Mikus E, Stepanenko A, Krabatsch T, Loforte A, Dandel M, Lehmkuhl HB, Hetzer R, Potapov EV (2011) Reversibility of fixed pulmonary hypertension in left ventricular assist device support recipients. Eur J Cardiothorac Surg 40(4):971–977. doi:10.1016/j.ejcts.2011.01.019
Salzberg SP, Lachat ML, von Harbou K, Zund G, Turina MI (2005) Normalization of high pulmonary vascular resistance with LVAD support in heart transplantation candidates. Eur J Cardiothorac Surg 27(2):222–225. doi:10.1016/j.ejcts.2004.11.001
Martin J, Siegenthaler MP, Friesewinkel O, Fader T, van de Loo A, Trummer G, Berchtold-Herz M, Beyersdorf F (2004) Implantable left ventricular assist device for treatment of pulmonary hypertension in candidates for orthotopic heart transplantation-a preliminary study. Eur J Cardiothorac Surg 25(6):971–977. doi:10.1016/j.ejcts.2004.01.052
Zimpfer D, Zrunek P, Roethy W, Czerny M, Schima H, Huber L, Grimm M, Rajek A, Wolner E, Wieselthaler G (2007) Left ventricular assist devices decrease fixed pulmonary hypertension in cardiac transplant candidates. J Thorac Cardiovasc Surg 133(3):689–695. doi:10.1016/j.jtcvs.2006.08.104
Guglin M, Verma S (2012) Right side of heart failure. Heart Fail Rev 17(3):511–527. doi:10.1007/s10741-011-9272-0
Boulate D, Marques MA, Ha R, Banerjee D, Haddad F (2014) Biventricular VAD versus LVAD for right heart failure. Annals of cardiothoracic surgery 3(6):585–588. doi:10.3978/j.issn.2225-319X.2014.08.08
Moazami N, Hill L (2003) Right ventricular dysfunction in patients with acute inferior MI: role of RV mechanical support. Thorac Cardiovasc Surg 51(5):290–292. doi:10.1055/s-2003-43079
Moazami N, Pasque MK, Moon MR, Herren RL, Bailey MS, Lawton JS, Damiano RJ Jr (2004) Mechanical support for isolated right ventricular failure in patients after cardiotomy. J Heart Lung Transplant 23(12):1371–1375. doi:10.1016/j.healun.2003.09.022
Haneya A, Philipp A, Puehler T, Rupprecht L, Kobuch R, Hilker M, Schmid C, Hirt SW (2012) Temporary percutaneous right ventricular support using a centrifugal pump in patients with postoperative acute refractory right ventricular failure after left ventricular assist device implantation. Eur J Cardiothorac Surg 41(1):219–223. doi:10.1016/j.ejcts.2011.04.029
Chen JM, Levin HR, Rose EA, Addonizio LJ, Landry DW, Sistino JJ, Michler RE, Oz MC (1996) Experience with right ventricular assist devices for perioperative right-sided circulatory failure. Ann Thorac Surg 61(1):305–310. doi:10.1016/0003-4975(95)01010-6 discussion 311–303
Takeda K, Naka Y, Yang JA, Uriel N, Colombo PC, Jorde UP, Takayama H (2014) Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J Heart Lung Transplant 33(2):141–148. doi:10.1016/j.healun.2013.06.025
Yerebakan C, Buz S, Huebler M, Weng Y, Lehmkuhl H, Hetzer R (2008) Right ventricular failure following heart transplantation–recovery after extended mechanical support. J Card Surg 23(5):578–580. doi:10.1111/j.1540-8191.2008.00698.x
Berman M, Tsui S, Vuylsteke A, Klein A, Jenkins DP (2008) Life-threatening right ventricular failure in pulmonary hypertension: RVAD or ECMO? J Heart Lung Transplant 27(10):1188–1189. doi:10.1016/j.healun.2008.07.017
Miller JR, Epstein DJ, Henn MC, Guthrie T, Schuessler RB, Simpson KE, Canter CE, Eghtesady P, Boston US (2015) Early biventricular assist device use in children: a single center review of 31 patients. ASAIO J. doi:10.1097/MAT.0000000000000268
Taghavi S, Zuckermann A, Ankersmit J, Wieselthaler G, Rajek A, Laufer G, Wolner E, Grimm M (2004) Extracorporeal membrane oxygenation is superior to right ventricular assist device for acute right ventricular failure after heart transplantation. Ann Thorac Surg 78(5):1644–1649. doi:10.1016/j.athoracsur.2004.04.059
Budev MM, Arroliga AC, Emery S (2005) Exacerbation of underlying pulmonary disease in pregnancy. Crit Care Med 33(10 Suppl):S313–S318
Martinez MV, Rutherford JD (2013) Pulmonary hypertension in pregnancy. Cardiol Rev 21(4):167–173. doi:10.1097/CRD.0b013e318275cf01
Weiss BM, Zemp L, Seifert B, Hess OM (1998) Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol 31(7):1650–1657
Bedard E, Dimopoulos K, Gatzoulis MA (2009) Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J 30(3):256–265. doi:10.1093/eurheartj/ehn597
Monnery L, Nanson J, Charlton G (2001) Primary pulmonary hypertension in pregnancy; a role for novel vasodilators. Br J Anaesth 87(2):295–298
Bossert T, Gummert JF, Bittner HB, Barten M, Walther T, Falk V, Mohr FW (2006) Swan-Ganz catheter-induced severe complications in cardiac surgery: right ventricular perforation, knotting, and rupture of a pulmonary artery. J Card Surg 21(3):292–295. doi:10.1111/j.1540-8191.2006.00235.x
Stewart R, Tuazon D, Olson G, Duarte AG (2001) Pregnancy and primary pulmonary hypertension: successful outcome with epoprostenol therapy. Chest 119(3):973–975
Easterling TR, Ralph DD, Schmucker BC (1999) Pulmonary hypertension in pregnancy: treatment with pulmonary vasodilators. Obstet Gynecol 93(4):494–498
Goland S, Tsai F, Habib M, Janmohamed M, Goodwin TM, Elkayam U (2010) Favorable outcome of pregnancy with an elective use of epoprostenol and sildenafil in women with severe pulmonary hypertension. Cardiology 115(3):205–208. doi:10.1159/000287638
Lam GK, Stafford RE, Thorp J, Moise KJ Jr, Cairns BA (2001) Inhaled nitric oxide for primary pulmonary hypertension in pregnancy. Obstet Gynecol 98(5 Pt 2):895–898
Robinson JN, Banerjee R, Landzberg MJ, Thiet MP (1999) Inhaled nitric oxide therapy in pregnancy complicated by pulmonary hypertension. Am J Obstet Gynecol 180(4):1045–1046
Decoene C, Bourzoufi K, Moreau D, Narducci F, Crepin F, Krivosic-Horber R (2001) Use of inhaled nitric oxide for emergency Cesarean section in a woman with unexpected primary pulmonary hypertension. Cana J Anaesth 48(6):584–587. doi:10.1007/BF03016836
Castro M, Krowka MJ, Schroeder DR, Beck KC, Plevak DJ, Rettke SR, Cortese DA, Wiesner RH (1996) Frequency and clinical implications of increased pulmonary artery pressures in liver transplant patients. Mayo Clin Proc 71(6):543–551. doi:10.1016/S0025-6196(11)64110-4
Cartin-Ceba R, Krowka MJ (2014) Portopulmonary hypertension. Clin Liver Dis 18(2):421–438. doi:10.1016/j.cld.2014.01.004
Ramsay M (2010) Portopulmonary hypertension and right heart failure in patients with cirrhosis. Curr Opin Anaesthesiol 23(2):145–150. doi:10.1097/ACO.0b013e32833725c4
Krowka MJ, Rodriguez-Roisin R (2012) Portopulmonary hypertension: a consequence of portal hypertension. Eur Respir Monogr 57:58–70. doi:10.1183/1025448x.10018811
Bozbas SS, Eyuboglu FO, Arslan NG, Ergur FO, Karakayali H, Haberal M (2009) The prevalence and the impact of portopulmonary hypertension on postoperative course in patients undergoing liver transplantation. Transplant Proc 41(7):2860–2863. doi:10.1016/j.transproceed.2009.06.178
Sakai T, Planinsic RM, Mathier MA, de Vera ME, Venkataramanan R (2009) Initial experience using continuous intravenous treprostinil to manage pulmonary arterial hypertension in patients with end-stage liver disease. Transpl Int 22(5):554–561. doi:10.1111/j.1432-2277.2008.00830.x
Hollatz TJ, Musat A, Westphal S, Decker C, D’Alessandro AM, Keevil J, Zhanhai L, Runo JR (2012) Treatment with sildenafil and treprostinil allows successful liver transplantation of patients with moderate to severe portopulmonary hypertension. Liver Transpl 18(6):686–695. doi:10.1002/lt.23407
Kahler CM, Graziadei I, Wiedermann CJ, Kneussl MP, Vogel W (2000) Successful use of continuous intravenous prostacyclin in a patient with severe portopulmonary hypertension. Wien Klin Wochenschr 112(14):637–640
Raevens S, De Pauw M, Reyntjens K, Geerts A, Verhelst X, Berrevoet F, Rogiers X, Troisi RI, Van Vlierberghe H, Colle I (2013) Oral vasodilator therapy in patients with moderate to severe portopulmonary hypertension as a bridge to liver transplantation. Eur J Gastroenterol Hepatol 25(4):495–502. doi:10.1097/MEG.0b013e32835c504b
Cartin-Ceba R, Swanson K, Iyer V, Wiesner RH, Krowka MJ (2011) Safety and efficacy of ambrisentan for the treatment of portopulmonary hypertension. Chest 139(1):109–114. doi:10.1378/chest.10-0574
Halank M, Knudsen L, Seyfarth HJ, Ewert R, Wiedemann B, Kolditz M, Hoffken G, Hoeper MM (2011) Ambrisentan improves exercise capacity and symptoms in patients with portopulmonary hypertension. Z Gastroenterol 49(9):1258–1262. doi:10.1055/s-0031-1273393
Reichenberger F, Voswinckel R, Steveling E, Enke B, Kreckel A, Olschewski H, Grimminger F, Seeger W, Ghofrani HA (2006) Sildenafil treatment for portopulmonary hypertension. Eur Respir J 28(3):563–567. doi:10.1183/09031936.06.00030206
Hemnes AR, Robbins IM (2009) Sildenafil monotherapy in portopulmonary hypertension can facilitate liver transplantation. Liver Transpl 15(1):15–19. doi:10.1002/lt.21479
Dalen JE, Dexter L, Ockene IS, Carlson J (1975) Precapillary pulmonary hypertension; its relationship to pulmonary venous hypertension. Trans Am Clin Climatol Assoc 86:207–218
Gerges M, Gerges C, Pistritto AMA, Lang MBM, Trip P, Jakowitsch J, Binder T, Lang IM (2015) Pulmonary hypertension in heart failure: epidemiology, right ventricular function and survival. Am J Respir Crit Care Med. doi:10.1164/rccm.201503-0529OC
Mancini D, Lietz K (2010) Selection of cardiac transplantation candidates in 2010. Circulation 122(2):173–183. doi:10.1161/CIRCULATIONAHA.109.858076
Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ (2007) Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 116(14):1555–1562. doi:10.1161/CIRCULATIONAHA.107.716373
Jiang R, Wang L, Zhu CT, Yuan P, Pudasaini B, Zhao QH, Gong SG, He J, Liu JM, Hu QH (2015) Comparative effectiveness of sildenafil for pulmonary hypertension due to left heart disease with HFrEF. Hypertens Res. doi:10.1038/hr.2015.73
Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, van Veldhuisen DJ, Voors AA (2015) Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J. doi:10.1093/eurheartj/ehv336
Bautin AE, Iakovlev AS, Tashkhanov DM, Datsenko SV, Marichev AO, Popov MA, Fedotov PA (2015) Specifics of inhaled iloprost pharmacodynamics in patients with severe left ventricular systolic dysfunction. Anesteziol Reanimatol 60(2):4–7
Sablotzki A, Czeslick E, Schubert S, Friedrich I, Muhling J, Dehne MG, Grond S, Hentschel T (2002) Iloprost improves hemodynamics in patients with severe chronic cardiac failure and secondary pulmonary hypertension. Can J Anaesth 49(10):1076–1080. doi:10.1007/BF03017906
Kalra PR, Moon JC, Coats AJ (2002) Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol 85(2–3):195–197
Kaluski E, Cotter G, Leitman M, Milo-Cotter O, Krakover R, Kobrin I, Moriconi T, Rainisio M, Caspi A, Reizin L, Zimlichman R, Vered Z (2008) Clinical and hemodynamic effects of bosentan dose optimization in symptomatic heart failure patients with severe systolic dysfunction, associated with secondary pulmonary hypertension—a multi-center randomized study. Cardiology 109(4):273–280. doi:10.1159/000107791
Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, Ruschitzka F, Luscher TF (2004) Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet 364(9431):347–354. doi:10.1016/S0140-6736(04)16723-8
McMurray JJ, Teerlink JR, Cotter G, Bourge RC, Cleland JG, Jondeau G, Krum H, Metra M, O’Connor CM, Parker JD, Torre-Amione G, van Veldhuisen DJ, Lewsey J, Frey A, Rainisio M, Kobrin I (2007) Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA 298(17):2009–2019. doi:10.1001/jama.298.17.2009
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39(2):165–228. doi:10.1007/s00134-012-2769-8
Guazzi M, Borlaug BA (2012) Pulmonary hypertension due to left heart disease. Circulation 126(8):975–990. doi:10.1161/CIRCULATIONAHA.111.085761
Grinnan DC, Swetz KM, Pinson J, Fairman P, Lyckholm LJ, Smith T (2012) The end-of-life experience for a cohort of patients with pulmonary arterial hypertension. J Palliat Med 15(10):1065–1070. doi:10.1089/jpm.2012.0085
Huynh TN, Weigt SS, Sugar CA, Shapiro S, Kleerup EC (2012) Prognostic factors and outcomes of patients with pulmonary hypertension admitted to the intensive care unit. J Crit Care 27(6):739 e7–739 e13. doi:10.1016/j.jcrc.2012.08.006
Myrianthefs P, Kalafati M, Lemonidou C, Minasidou E, Evagelopoulou P, Karatzas S, Baltopoulos G (2003) Efficacy of CPR in a general, adult ICU. Resuscitation 57(1):43–48
Hoeper MM, Galie N, Murali S, Olschewski H, Rubenfire M, Robbins IM, Farber HW, McLaughlin V, Shapiro S, Pepke-Zaba J, Winkler J, Ewert R, Opitz C, Westerkamp V, Vachiery JL, Torbicki A, Behr J, Barst RJ (2002) Outcome after cardiopulmonary resuscitation in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 165(3):341–344. doi:10.1164/ajrccm.165.3.200109-0130c
Fenstad ER, Shanafelt TD, Sloan JA, Novotny PJ, Durst LA, Frantz RP, McGoon MD, Swetz KM (2014) Physician attitudes toward palliative care for patients with pulmonary arterial hypertension: results of a cross-sectional survey. Pulm Circ 4(3):504–510. doi:10.1086/677365
Swetz KM, Shanafelt TD, Drozdowicz LB, Sloan JA, Novotny PJ, Durst LA, Frantz RP, McGoon MD (2012) Symptom burden, quality of life, and attitudes toward palliative care in patients with pulmonary arterial hypertension: results from a cross-sectional patient survey. J Heart Lung Transplant 31(10):1102–1108. doi:10.1016/j.healun.2012.08.010
Haddad F, Hunt SA, Rosenthal DN, Murphy DJ (2008) Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 117(11):1436–1448. doi:10.1161/CIRCULATIONAHA.107.653576
Kaul S, Tei C, Hopkins JM, Shah PM (1984) Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J 107(3):526–531
Leather HA, Ama R, Missant C, Rex S, Rademakers FE, Wouters PF (2006) Longitudinal but not circumferential deformation reflects global contractile function in the right ventricle with open pericardium. Am J Physiol Heart Circ Physiol 290(6):H2369–H2375. doi:10.1152/ajpheart.01211.2004
Haeck ML, Scherptong RW, Marsan NA, Holman ER, Schalij MJ, Bax JJ, Vliegen HW, Delgado V (2012) Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ Cardiovasc Imaging 5(5):628–636. doi:10.1161/CIRCIMAGING.111.971465
Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, Kane GC (2013) Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Cir Cardiovasc Imaging 6(5):711–721. doi:10.1161/CIRCIMAGING.113.000640
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coz Yataco, A., Aguinaga Meza, M., Buch, K.P. et al. Hospital and intensive care unit management of decompensated pulmonary hypertension and right ventricular failure. Heart Fail Rev 21, 323–346 (2016). https://doi.org/10.1007/s10741-015-9514-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-015-9514-7