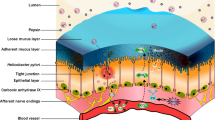
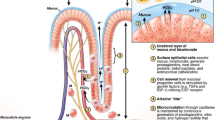
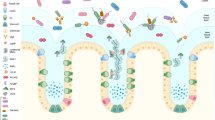
Abstract
Of the numerous gaseous substances that can act as signaling molecules, the best characterized are nitric oxide, carbon monoxide and hydrogen sulfide. Contributions of each of these low molecular weight substances, alone or in combination, to maintenance of gastrointestinal mucosal integrity have been established. There is considerable overlap in the actions of these gases in modulating mucosal defense and responses to injury, and in some instances they act in a cooperative manner. Each also play important roles in regulating inflammatory and repair processes throughout the gastrointestinal tract. In recent years, significant progress has been made in the development of novel anti-inflammatory and cytoprotective drugs that exploit the beneficial activities of one or more of these gaseous mediators.


Similar content being viewed by others
References
Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88:1547–1565.
Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142.
Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071.
Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41:113–121.
Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016.
Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015;14:329–345.
Olson KR, Straub KD. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology. 2016;31:60–72.
Goubern M, Andriamihaja M, Nübel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21:1699–1706.
Mimoun S, Andriamihaja M, Chaumontet C, et al. Detoxification of H2S by differentiated colonic epithelial cells: implication of the sulfide oxidizing unit and of the cell respiratory capacity. Antioxid Redox Signal. 2012;17:1–10.
Motterlini R, Foresti R. Biological signaling by carbon monoxide and carbon monoxide-releasing molecules. Am J Physiol Cell Physiol. 2017;312:C302–C313.
Chang M, Xue J, Sharma V, Habtezion A. Protective role of hemeoxygenase-1 in gastrointestinal diseases. Cell Mol Life Sci. 2015;72:1161–1173.
Gibbons SJ, Verhurst PJ, Bharucha A, Farrugia G. Review article: carbon monoxide in gastrointestinal physiology and its potential in therapeutics. Aliment Pharmacol Ther. 2013;38:689–702.
Farugia G, Szurszewski JH. Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology. 2014;147:303–313.
Yoda Y, Amagase K, Kato S, et al. Prevention by lansoprazole, a proton pump inhibitor, of indomethacin-induced small intestinal ulceration in rats through induction of heme oxygenase-1. J Physiol Pharmacol. 2010;61:287–294.
Brown JF, Hanson PJ, Whittle BJ. Nitric oxide donors increase mucus gel thickness in rat stomach. Eur J Pharmacol. 1992;223:103–104.
Price KJ, Hanson PJ, Whittle BJR. Stimulation by carbachol of mucus gel thickness in rat stomach involves nitric oxide. Eur J Pharmacol. 1994;263:199–202.
Lucetti LT, Silva RO, Santana AP, et al. Nitric oxide and hydrogen sulfide interact when modulating gastric physiological functions in rodents. Dig Dis Sci. 2017;62:93–104.
Costa NR, Silva RO, Nicolau LA, et al. Role of soluble guanylate cyclase activation in the gastroprotective effect of the HO-1/CO pathway against alendronate-induced gastric damage in rats. Eur J Pharmacol. 2013;700:51–59.
Motta JP, Flannigan KL, Agbor TA, et al. Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflamm Bowel Dis. 2015;21:1006–1017.
Perdue MH, McKay DM. Integrative immunophysiology in the intestinal mucosa. Am J Physiol. 1994;267:G151–G165.
MacNaughton WK. Nitric oxide-donating compounds stimulate electrolyte transport in the guinea pig intestine in vitro. Life Sci. 1993;53:585–593.
Tamai H, Gaginella TS. Direct evidence for nitric oxide stimulation of electrolyte secretion in the rat colon. Free Radic Res. 1993;19:229–239.
Asfaha S, MacNaughton WK, Appleyard CB, Chadee K, Wallace JL. Persistent epithelial dysfunction and bacterial translocation after resolution of intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G635–G644.
Asfaha S, Bell CJ, Wallace JL, MacNaughton WK. Prolonged colonic epithelial hyperresponsiveness after colitis: role of inducible nitric oxide synthase. Am J Physiol. 1999;276:G703–G710.
Takeuchi K, Aihara E, Kimura M, Dogishi K, Hara T, Hayashi S. Gas mediators involved in modulating duodenal HCO3 secretion. Curr Med Chem. 2012;19:43–54.
Takasuka H, Hayashi S, Koyama M, et al. Carbon monoxide involved in modulating HCO -3 secretion in rat duodenum. J Pharmacol Exp Ther. 2011;337:293–300.
Takeuchi K, Kita K, Hayashi S, Aihara E. Regulatory mechanism of duodenal bicarbonate secretion: roles of endogenous prostaglandins and nitric oxide. Pharmacol Ther. 2011;130:59–70.
Blackler R, Syer S, Bolla M, Ongini E, Wallace JL. Gastrointestinal-sparing effects of novel NSAIDs in rats with compromised mucosal defence. PLoS ONE. 2012;7:e35196.
Mard SA, Askari H, Neisi N, Veisi A. Antisecretory effect of hydrogen sulfide on gastric acid secretion and the involvement of nitric oxide. Biomed Res Int. 2014;2014:480921.
Verdu E, Viani F, Armstrong D, et al. Effect of omeprazole on intragastric bacterial counts, nitrates, nitrites, and N-nitroso compounds. Gut. 1994;35:455–460.
Imhann F, Vich Vila A, Bonder MJ, et al. The influence of proton pump inhibitors and other commonly used medication on the gut microbiota. Gut Microbes. 2017;24:1–8.
Keszthelyi D, Jansen SV, Schouten GA, et al. Proton pump inhibitor use is associated with an increased risk for microscopic colitis: a case-control study. Aliment Pharmacol Ther. 2010;32:1124–1128.
Shah R, Richardson P, Yu H, Kramer J, Hou JK. Gastric acid suppression is associated with an increased risk of adverse outcomes in inflammatory bowel disease. Digestion. 2017;95:188–193.
Fujimori S. What are the effects of proton pump inhibitors on the small intestine? World J Gastroenterol. 2015;21:6817–6819.
Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–1322.
Watanabe T, Tanigawa T, Nadatani Y, et al. Risk factors for severe nonsteroidal anti-inflammatory drug-induced small intestinal damage. Dig Liver Dis. 2013;45:390–395.
Washio E, Esaki M, Maehata Y, et al. Proton pump inhibitors increase incidence of nonsteroidal anti-inflammatory drug-induced small bowel injury: a randomized placebo-controlled trial. Clin Gastroenterol Hepatol. 2016;14:809–815.
Nuki Y, Umeno J, Washio E, et al. The influence of CYP2C19 polymorphisms on exacerbating effect of rabeprazole in celecoxib-induced small bowel injury. Aliment Pharmacol Ther. 2017. doi:10.1111/apt.14134.
Blackler RW, De Palma G, Manko A, et al. Deciphering the pathogenesis of NSAID enteropathy using proton pump inhibitors and a hydrogen sulfide-releasing NSAID. Am J Physiol Gastrointest Liver Physiol. 2015;308:G994–G1003.
Blackler RW, Motta JP, Manko A, et al. Hydrogen sulphide protects against NSAID-enteropathy through modulation of bile and the microbiota. Br J Pharmacol. 2015;172:992–1004.
Kidder GW. Carbon monoxide insensitivity of gastric acid secretion. Am J Physiol. 1980;238:G197–G202.
Pique JM, Esplugues JV, Whittle BJ. Endogenous nitric oxide as a mediator of gastric mucosal vasodilatation during acid secretion. Gastroenterology. 1992;102:168–174.
Holzer P, Sametz W. Gastric mucosal protection against ulcerogenicfactors in the rat mediated by capsaicin-sensitive afferent neurons. Gastroenterology. 1986;91:975–981.
Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839.
Lippe IT, Holzer P. Participation of endothelium-derived nitric oxide but not prostacyclin in the gastric mucosal hyperaemia due to acid back-diffusion. Br J Pharmacol. 1992;105:708–714.
Fiorucci S, Antonelli T, Distrutti E, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory non-steroidal drugs. Gastroenterology. 2005;129:1210–1224.
Magierowska K, Magierowski M, Hubalewska-Mazgaj M, et al. Carbon monoxide (CO) released from tricarbonyldichlororuthenium (II) dimer (CORM-2) in gastroprotection against experimental ethanol-induced gastric damage. PLoS One. 2015;10:e0140493.
Magierowski M, Magierowska K, Hubalewska-Mazgaj M, et al. Interaction between endogenous carbon monoxide and hydrogen sulfide in the mechanism of gastroprotection against acute aspirin-induced gastric damage. Pharmacol Res. 2016;114:235–250.
Banick PD, Chen QP, Xu YA, Thom SR. Nitric oxide inhibits neutrophil b2 integrin function by inhibiting membrane-associated cyclic GMP synthesis. J Cell Physiol. 1997;172:12–24.
Davenpeck KL, Gauthier TW, Lefer AM. Inhibition of endothelial derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation. Gastroenterology. 1994;107:1050–1058.
Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655.
Wallace JL, Vergnolle N, Muscara MN, et al. Enhanced anti-inflammatory effects of a nitric oxide-releasing derivative of mesalamine in rats. Gastroenterology. 1999;117:557–566.
Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120.
Wallace JL, Caliendo G, Santagada V, Cirino G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346). Br J Pharmacol. 2010;159:1236–1246.
Urquhart P, Rosignoli G, Cooper D, Motterlini R, Perretti M. Carbon monoxide-releasing molecules modulate leukocyte-endothelial interactions under flow. J Pharmacol Exp Ther. 2007;321:656–662.
Paul G, Bataille F, Obermeier F, et al. Analysis of intestinal haem-oxygenase-1 (HO-1) in clinical and experimental colitis. Clin Exp Immunol. 2005;140:547–555.
Tepperman BL, Soper BD. Nitric oxide synthase induction and cytoprotection of rat gastric mucosa from injury by ethanol. Can J Physiol Pharmacol. 1994;72:1308–1312.
Motterlini R, Foresti R, Intaglietta M, Winslow RM. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am J Physiol. 1996;270:H107–H114.
Lohmander LS, McKeith D, Svensson O, et al. Ramos-Remus C; STAR Multinational Study Group. A randomised, placebo controlled, comparative trial of the gastrointestinal safety and efficacy of AZD3582 versus naproxen in osteoarthritis. Ann Rheum Dis. 2005;64:449–456.
Wallace JL. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid Redox Signal. 2010;12:1125–1133.
Wallace JL, Vaughan D, Dicay M, MacNaughton WK, de Nucci G. Hydrogen sulfide-releasing therapeutics: Translation to the clinic. Antiox Redox Signal 2017; in press (doi: 10.1089/ars.2017.7068).
Uc A, Zhu X, Wagner BA, Guettner GR, Berg DJ. Heme oxygenase-1 is protective against nonsteroidal anti-inflammatory drug-induced gastric ulcers. J Ped Gastroenterol Nutr. 2012;54:471–476.
Cheng Y-T, Wu C-H, Ho C-Y, Yen G-C. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J Nutr Biochem. 2013;24:475–483.
De Backer O, Elinck E, Blanckaert B, Leybaert L, Motterlini R, Lefebvre RA. Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signalling and reduction of oxidative stress. Gut. 2009;58:347–356.
Konturek SJ, Brzozowski T, Majka J, Pytko-Polonczyk J, Stachura J. Inhibition of nitric oxide synthase delays healing of chronic gastric ulcers. Eur J Pharmacol. 1993;239:215–217.
Elliott SN, McKnight W, Cirino G, Wallace JL. A nitric oxide-releasing nonsteroidal anti-inflammatory drug accelerates gastric ulcer healing in rats. Gastroenterology. 1995;109:524–530.
Wallace JL, Dicay M, McKnight W, Martin GR. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–4076.
Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137:569–578.
Flannigan KL, Ferraz JGP, Wang R, Wallace JL. Enhanced synthesis and diminished degradation of hydrogen sulfide in experimental colitis: A site-specific, pro-resolution mechanism. PLoS One. 2013;8:e71962.
Takagi T, Naito Y, Uchiyama K, et al. Carbon monoxide promotes gastric wound healing in mice via the protein kinase C pathway. Free Radic Res. 2016;50:1098–1105.
Schaffer MR, Tantry U, Gross SS, Wasserkrug HL, Barbul A. Nitric oxide regulates wound healing. J Surg Res. 1996;63:237–240.
Hirose H, Takeuchi K, Okabe S. Effect of indomethacin on gastric mucosal blood flow around acetic acid–induced gastric ulcers in rats. Gastroenterology. 1991;100:1259–1265.
Papapetropoulos A, Foresti R, Ferdiandy P. Pharmacology of the ‘gasotransmitters’ NO, CO and H2S: translational opportunities. Br J Pharmacol. 2015;172:1395–1396.
Flannigan KL, Agbor TA, Blackler RW, et al. Impaired hydrogen sulfide synthesis and IL-10 signaling underlie hyperhomocysteinemia-associated exacerbation of colitis. Proc Natl Acad Sci USA. 2014;111:13559–13564.
Lagoutte E, Mimoun S, Andriamihaja M, et al. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta. 2010;1797:1500–1511.
Shen X, Carlström M, Borniquel S, Jädert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med. 2013;60:195–200.
Flannigan KL, McCoy KD, Wallace JL. Eukaryotic and prokaryotic contributions to colonic hydrogen sulfide synthesis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G188–G193.
Lund JN, Scholefield JH. Glyceryl trinitrate is an effective treatment for anal fissure. Dis Colon Rectum. 1997;40:468–470.
Hogaboam CM, Jacobson K, Collins SM, Blennerhassett MG. The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am J Physiol. 1995;268:G673–G684.
Aiko S, Fuseler J, Grisham MB. Effects of nitric oxide synthase inhibition or sulfasalazine on the spontaneous colitis observed in HLA-B27 transgenic rats. J Pharmacol Exp Ther. 1998;284:722–727.
Soufli I, Toumi R, Rafa H, Touil-Boukoffa C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016;7:353–360.
McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022–1027.
Takagi T, Naito Y, Uchiyama K, et al. Carbon monoxide liberated from carbon monoxide-releasing molecule exerts an anti-inflammatory effect on dextran sulfate sodium-induced colitis in mice. Dig Dis Sci. 2011;56:1663–1671.
Uddin MJ, Jeong SO, Zheng M, et al. Carbon monoxide attenuates dextran sulfate sodium-induced colitis via inhibition of GSK-3ß signaling. Oxid Med Cell Longev. 2013;2013:210563.
Megias J, Busserolles J, Alcaraz MJ. The carbon monoxide-releasing molecule CORM-2 inhibits the inflammatory response induced by cytokines in Caco-2 cells. Br J Pharmacol. 2007;150:977–986.
Onyiah JC, Sheikh SZ, Maharshak N, Otterbein LE, Plevy SE. Heme oxygenase-1 and carbon monoxide regulate intestinal homeostasis and mucosal immune responses to the enteric microbiota. Gut Microbes. 2014;5:220–224.
Szczesny B, Módis K, Yanagi K, et al. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide. 2014;41:120–130.
Shibuya N, Koike S, Tanaka M, et al. A novel pathway for the production of hydrogen sulfide from d-cysteine in mammalian cells. Nat Commun. 2013;4:1366.
Wallace JL, Blackler RW, Chan MV, et al. Anti-inflammatory and cytoprotective actions of hydrogen sulfide: translation to therapeutics. Antiox Redox Signal. 2015;22:398–410.
Acknowledgments
Dr. Wallace’s research is supported by a grant from the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Wallace is the founder and Chief Scientific Officer of Antibe Therapeutics Inc., which is developing hydrogen sulfide-releasing drugs.
Rights and permissions
About this article
Cite this article
Wallace, J.L., Ianaro, A. & de Nucci, G. Gaseous Mediators in Gastrointestinal Mucosal Defense and Injury. Dig Dis Sci 62, 2223–2230 (2017). https://doi.org/10.1007/s10620-017-4681-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4681-0